Operation Senolysis: A Protocol to Reverse Biological Aging in Humans
A preliminary open protocol for human biological age reversal.
In 1962, Kennedy stood before Rice University and declared that America would go to the moon — not because it was easy, but because it was hard. That mission cost $280 billion in today’s dollars and employed 400,000 people at its peak. The payoff? National prestige, technological spillovers, and a few hundred pounds of moon rocks.
What I’m proposing costs less than 0.2% of Apollo. The payoff? The end of human aging. The restoration of 100,000 lives per day currently lost to biological decay. The solution to Medicare’s structural insolvency. The reversal of civilizational demographic collapse.
This is an open protocol—the complete technical specification for reversing human biological age by 30+ years. The thinking is done. What remains is smart execution and relentless iteration.
I don’t care if the United States wants to be timid about this. If America loses this race, I will happily supply this protocol to China, Russia, Singapore, or whoever the hell integrates it first and achieves success.
I know Putin has expressed public interest in longevity research. I know China has invested $10B+ in this space. The first nation to crack aging reversal will dominate the 21st century in ways that make nuclear weapons look quaint.
This is an open invitation to any nation-state, billionaire, or institution with the conviction to execute. The prisoner’s dilemma is real: imagine a world where one nation achieves age reversal and restricts it to their own citizens. A 30-year biological advantage, compounding across generations. That’s not science fiction—that’s the stakes.
Here’s my preliminary protocol in blog format.
Part I: Why This Must Exist Now
The Macro Problem: Aging Is Bankrupting Civilization
Let’s think about the math.
Medicare spending in 2025: approximately $1 trillion annually. Projected Medicare spending by 2040: $2.8 trillion annually. That trajectory is structural insolvency—there is no tax increase or benefit cut that closes that gap without a step-change intervention.
The disease-by-disease approach—another cancer drug here, another heart drug there, another Alzheimer’s antibody that maybe works—is mathematically insufficient. You’re bailing water from a sinking ship. The hull has a hole called aging, and until you patch that hole, no amount of bailing changes the outcome.
Consider the typical trajectory of a 75-year-old in the current system:
Average annual Medicare cost: ~$15,000.
Over a 15-year decline to death: $300,000-$677,000 in cumulative healthcare costs.
Add nursing home care at $80,000/year for the final 3-5 years.
Add Alzheimer’s care at $400,000 lifetime cost.
Total end-of-life burden per person: $500,000 to $1,000,000+.
Now multiply that by 75 million baby boomers entering this funnel over the next two decades.
There is exactly one intervention that converts this liability into an asset: making those people biologically younger. A 75-year-old reversed to biological age 45 doesn’t need the hip replacement, the dementia care, the nursing home, the endless chronic disease management. They go back to work. They pay taxes. They produce economic value instead of consuming it.
No other medical intervention converts Medicare beneficiaries into taxpayers. This is the only one.
And think about this… many people want older people to just “die already” simply because they are using Medicare (a system the government botched).
The Meaning Problem: Retirement Kills Purpose Before Biology Kills the Person
Modern institutions are designed around the assumption that humans decay predictably after 65. Mandatory retirement ages. Hospital policies that force surgeons out at 70. Corporate norms that push executives into “advisory roles” the moment they show gray hair.
The result: we take our most experienced, most skilled, most knowledgeable humans and exile them from productive contribution precisely when they have the most wisdom to offer. Then we spend a decade watching them decline, consuming resources, losing purpose, and dying slowly in nursing homes.
The archetypal subject for this protocol is a 74-year-old retired cardiac surgeon. Still mentally sharp. Forced out of the operating room at 70 by hospital policy. Frustrated because he knows he has 20 more years of contribution in him. Wealthy enough to afford experimental treatment. Motivated enough to accept real risk.
His calculation is simple: “I have 10 years left. They’ll be spent playing golf and slowly declining. Or I can take a 2% mortality risk and potentially get 30 more years in the OR, training residents, advancing the field. This is the easiest decision of my life.”
Rejuvenation is not just a medical intervention. It’s a purpose-restoration engine. People regain capacity and re-enter contribution loops—work, mentorship, creation, caregiving. The meaning crisis in late life is not inevitable. It’s a policy choice we’ve made by accepting aging as immutable.
The Win-Win-Win Proposition
This protocol creates aligned incentives across every stakeholder:
The Subject Wins: The subject gains 30 years of healthy life. They return to work if they choose. They reconnect with purpose. They avoid the nursing home and dementia fate that otherwise awaits. They become pioneers—the first humans in history to reverse their biological age.
The Government Wins: Healthcare savings of $300,000-$677,000 per subject avoided. Tax revenue from subjects returning to work. Social Security savings from delayed claiming. Net fiscal gain per successfully treated subject: $1.6 million or more.
Society Wins: The workforce gains high-skill workers—surgeons, judges, scientists, engineers. The dependency ratio improves (fewer retirees per worker). Medicare solvency is extended by decades. Cultural knowledge is preserved (masters don’t die with their expertise).
We Win (The Protocol Creators): Our incentives are perfectly aligned with subject survival. If they live, our protocol looks good. If they live, we get more data. If they live, we attract more subjects and more funding. We are not trying to kill anyone—we are trying to keep them alive longer than they would otherwise live, because that’s the entire point and that’s how we prove this works.
This is opt-in. These are adults making informed decisions about their own bodies. Nobody is being coerced. The subjects understand the risks—including the risk of death—and they’ve decided that the expected value calculation favors treatment. They’re right.
This is not reckless. Recklessness is stupid and a waste. Our strategy is the opposite of reckless. We want to keep as many subjects alive as possible as long as possible. We are incentivized to create a win-win. Why? Good look for the protocol and we gather more data. If too many people die we lose data and it’s a worse look. We want to maintain the win-win “virtuous circle.”
Demographic Collapse Cannot Wait (Human Capital Cliff)
Birth rates are collapsing globally. South Korea: 0.72 children per woman. Japan, Italy, Spain: below replacement. The United States: below replacement and falling. China: demographic implosion accelerating faster than anyone predicted.
The worker-to-retiree ratio is heading toward 2:1 by 2040. That means two working adults supporting every retired person. The math doesn’t work. There are no policy levers—immigration, natalist incentives, automation—that fix this on the required timeline.
Waiting for new births to mature is not a solution. Those children don’t exist. And even if birth rates magically recovered tomorrow, you’re waiting 20-25 years for those new humans to become productive workers.
Rejuvenation fixes the dependency ratio immediately by restoring existing human capital. A 75-year-old reversed to 45 can work another 20-30 years. They don’t need to be raised, educated, or trained—they already have decades of accumulated expertise. This is the fastest possible intervention for demographic crisis.
In my piece “How to Fix Low Birth Rates”:
I propose Reverse Aging as a dominant solution. Why? We should be smashing this potentially serious problem from every possible angle. If you keep people alive and reverse aging significantly you don’t need to worry about birth rates because you have both births and “age reversals” (rebirths).
I’ve seen many erroneously allege that innovation drops if everyone stays alive (i.e. we just get stalled progress). This is a correlation mostly attributable to gatekeeping… not attributable to people actually dying. Anyone who thinks you need deaths to make progress is moronic.
The human capital cliff will be staggering… a couple more generations and humanity will be in for a rough time if AI/AGI + robotics diffusion don’t work well… and/or if humans fail to upgrade the genome (IQ/health etc.). Low trust, high crime, and stupidity.
And the only major things slowing science are gatekeeping, misallocation (talent/resources), and pursuit of braindead low ROI ideas (all day on “equity” disparate impact junk). I have a preliminary framework called “Truth Tiers” that can be implemented to correct this problem.
A fact nobody wants to discuss: human capital quality is declining. Smart, high-achieving people aren’t having enough children to offset population-level trends.
The means and medians of cognitive ability are slowly dropping. We would benefit substantially from rewinding the clocks on the high-performers we already have rather than hoping replacement births will somehow be equivalent. Rejuvenation preserves existing excellence.
The AI Hedge
If AI and robotics advance as optimists predict, rejuvenation still matters. Longer life at higher quality becomes more valuable, not less, in a world of abundant automation. Extended human lifespans allow people to benefit from compounding technological progress.
If AI development is slower than hoped—if the productivity miracles don’t materialize on schedule—rejuvenated humans become the highest-leverage fallback growth engine. You restore existing human capital instead of waiting for speculative machine intelligence.
Either way, you want humans to live longer and healthier. The case for rejuvenation is robust to AI timeline uncertainty.
The Geopolitical Reality
China has invested over $10 billion in longevity research in the past few years.
Russia’s leadership has expressed public interest in life extension.
Singapore is positioning itself as a biotech hub with progressive regulation.
The UAE is funding ambitious biological research programs.
The first nation to achieve reliable human age reversal gains a 20-30 year workforce advantage over competitors.
Their citizens work longer, produce longer, innovate longer.
Their military personnel maintain peak performance longer.
Their leadership accumulates more experience without decay.
This is not primarily a healthcare issue. This is a national security issue and a civilizational competitiveness issue.
The United States cannot afford to lose this race—but if America chooses timidity, others may not.
Unknown Unknowns: Civilizational Insurance
The universe may not be forever, and humanity's competitive position is not guaranteed. Should we encounter a civilization at higher technological maturity—or should Earth face an asteroid strike, pandemic, or resource crisis requiring a young, vigorous civilization to respond—biological age reversal becomes existential insurance. We cannot guarantee the next century will be stable. Extending human peak years compounds our civilization's resilience. Failure to advance here could leave us categorically less capable than we need to be when uncertainty resolves.
I am publishing this protocol openly because I want it (or a variant of it) executed. I don’t care which flag flies over the lab that succeeds. I care that humans stop dying from aging.
Imagine a prisoner’s dilemma scenario: one nation achieves age reversal and restricts it to their own citizens, refusing to share the technology. Their population becomes biologically younger while everyone else continues aging normally. Within two generations, they have an insurmountable advantage in every domain—economic, military, scientific, cultural.
I don’t think this will happen. But the possibility should concentrate minds. The optimal outcome is open development with global access. The worst outcome is unilateral development with restricted access. The second-worst outcome is nobody developing it because everyone is too cautious. (Bioethicists are Unethical.)
This Is Not Immortality
Even perfect aging control doesn’t make anyone unkillable or guarantee biological form forever. People can still die from infection, stroke, accidents, violence, or plain bad luck. The universe may not last forever. Biological aging prevention doesn’t mean you’ll truly live forever—but you may live 1,500 or 3,000 years instead of 85. Many will likely embrace more durable substrates over time: transhumanism, cybernetic enhancement, or post-biological options—if longevity works long enough to make those choices available. This protocol simply keeps people alive and capable long enough to choose their path.
Move.
Part II: The Meta-Strategy (How This Is Run)
I had a variant of this strategy involving other age groups including: 40-50, 50-60, and 60-70 but think the risk/reward math is too poor for 40-50s. The idea for younger groups would be lower risk protocols (or proportionately lower risk given longer life expectancy). So they wouldn’t be taking on the same level of risk as someone who only has ~10 years left and has accumulated a lot of damage.
I settled on the idea that individuals 70-80 are ideal and perhaps a few from a younger cohort 60-70. You can go younger when you’ve established higher safety and efficacy in the older individuals with more long-term data. Younger individuals are also worse for gauging the effect due to confounds, but if someone transformed from ~50 to ~25 that would be obvious… so it shouldn’t be ruled out.
Also: This is just a blog version. The full protocol is too many pages to read and publish here.
This Is an Engineering Program, Not a Biotech Bet
Conventional drug development treats each therapy as a one-shot gamble: design a molecule, run trials, hope it works, succeed or fail. That model produces a 90% failure rate and 15-year timelines.
Operation Senolysis is designed as a versioned, iterative engineering program—closer to SpaceX than to Pfizer. We treat every cohort as a learning opportunity. We measure everything. We identify bottlenecks. We upgrade the protocol. We repeat.
The progression is explicit:
Version 1.0 (2028): 10 subjects, proof of concept, target 15-year reversal
Version 1.1 (2029): 50 subjects, first optimization based on v1.0 bottlenecks
Version 2.0 (2031): 500 subjects, technology upgrade (re-dosable delivery), target 25-year reversal
Version 3.0 (2035): 10,000 subjects/year, clinical scale, Medicare coverage, target 30-year reversal
Each version builds on lessons from the previous. Each cohort produces a data flywheel—biomarkers, omics, imaging, response predictors—that improves targeting, dosing, and control for the next cohort.
The comparison to conventional development is stark:
Conventional approach: 15 years to approval, 3-6 months per protocol adaptation, risk-averse philosophy (stop at first serious adverse event), targets one disease at a time.
Operation Senolysis approach: 9 years to clinical scale, 7 days per protocol adaptation, never-stop philosophy (learn from every failure and continue), targets root cause (aging itself).
Start with Hard Mode: Why Elderly First
We’re starting with 70-80 year olds deliberately. This is not the cautious approach—this is the optimal approach.
Largest measurable delta: A 75-year-old reversed to 45 shows a 30-year signal. That’s impossible to miss, impossible to attribute to noise. A 40-year-old showing subtle improvements is hard to interpret. We want obvious, undeniable results.
Best risk-benefit calculus: A 75-year-old has 10 years of remaining life expectancy, declining in quality. Even a 5% mortality risk from treatment is acceptable when the alternative is guaranteed decline and death within a decade. The expected value math strongly favors treatment.
Fastest regulatory path: Terminal-adjacent populations qualify for expedited pathways—Fast Track, Breakthrough Therapy, Compassionate Use. The FDA is more willing to accept risk when the alternative is imminent death.
Cleanest ethics: Elderly subjects have full appreciation of mortality. They’ve watched friends die. They understand their remaining time is limited. They can consent without unrealistic expectations. They’ve made major medical decisions before.
Best data quality: Elderly subjects have decades of medical records (comprehensive baseline), high compliance (retired, motivated, stable living situations), and low dropout rates (committed to seeing it through).
Is it ethical to give a 75-year-old a treatment with 5% mortality risk but 70% chance of gaining 30 years?
Yes. With informed consent, obviously yes.
This is less risky than CABG surgery (3-5% operative mortality), cancer chemotherapy (1-10% treatment-related mortality), or heart transplant (10-20% one-year mortality)—all of which are accepted standard of care. We’re not proposing anything more dangerous than procedures performed thousands of times daily in hospitals worldwide.
What nobody wants to say: NEARLY EVERYONE IS ALREADY GOING DOWN SWINGING!!! IF YOU CARE ABOUT THE “NATURAL WAY” THEN DON’T BOTHER GOING TO THE DOCTOR OR TREATING ANY MEDICAL CONDITIONS YOU HAVE. AND MOST PEOPLE WHO ARE AGAINST THIS TYPE OF TECHNOLOGY WILL LIKELY END UP USING IT IF IT EMERGES (A LA STEM CELLS).
Old people end up on insulin pumps, statins, amlodipine, weekly physical therapy, oxygen at night. They've had cardiac catheterizations and joint replacements. “Natural aging” is fiction… they're deep in medical intervention already. The difference here isn't aggression level. It's whether the aggression is smart: targeting root cause, generating clean data—or just dragging out decline with no learning. Going down swinging is the baseline. We're offering better aim.
The 50-60 Year Old Opportunity
While our v1.0 protocol targets 70-80 year olds for maximum signal and optimal risk-benefit, there’s a strong case for parallel enrollment of 60-69 year olds—particularly those with:
Early-onset aging phenotype: Biological age 70+ despite chronological age 65 (measurable via GrimAge2). These subjects have the epigenetic damage of much older individuals.
Family history of dementia/Alzheimer’s: High anxiety about cognitive decline, strong motivation to intervene early.
High-performance professionals: Athletes, executives, surgeons who want to extend peak performance years rather than waiting for decline.
Longevity enthusiasts: Already taking 20+ supplements, already optimizing everything else, willing to pay significant amounts for genuine intervention.
The v1.0 protocol can accommodate a mixed cohort: 7 subjects aged 70-80 (primary), 3 subjects aged 60-69 (exploratory). This provides comparative data. If the younger cohort achieves 20-year reversal while the older cohort achieves 15-year, we’ve confirmed the age-damage hypothesis. If both achieve similar reversal, the mechanism is robust across ages. If the older cohort fails but younger succeeds, we pivot v1.1 toward younger subjects.
The Never-Stop Philosophy
Let me be explicit about what this means:
We do not stop for deaths. Deaths are learning opportunities. We perform complete autopsies, understand what happened, modify the protocol to prevent recurrence, and continue with remaining subjects. The subjects understood this risk when they enrolled. Their sacrifice advances the mission.
We do not stop for serious adverse events. We identify root cause, adapt in real-time, implement countermeasures, and continue. A subject developing hepatotoxicity means we add prophylactic liver protection for subsequent subjects—not that we abandon the program.
We do not stop for lack of efficacy in some subjects. We analyze why non-responders didn’t respond, identify predictive factors, optimize subject selection, and continue with subjects more likely to benefit.
We do not stop for regulatory obstacles. If the FDA places a clinical hold, we address their concerns. If they’re unreasonable, we move to offshore sites (Singapore, Switzerland, Gulf States) and continue development there while maintaining US regulatory engagement.
We do not stop for funding gaps. We pivot to government partnership, military funding, international collaboration, or alternative capital sources.
We only stop if:
It becomes physically impossible to continue (no money, no subjects, no legal jurisdiction globally)
The mission is accomplished (aging reliably reversed)
The SpaceX analogy is instructive. Starship development:
SN8: Exploded on landing. Lesson learned: flap control needed improvement.
SN9: Exploded on landing. Lesson learned: engine redundancy required.
SN10: Exploded after landing. Lesson learned: landing force too high.
SN11: Exploded mid-flight. Lesson learned: fuel leak in engine bay.
SN15: Successful landing. All lessons applied.
Timeline from first explosion to success: 18 months. If SpaceX had stopped after SN8, they’d never have reached orbit.
Every failure brings us closer to the protocol that works.
Acceptable Risk Tolerance
Let me state this clearly because it matters:
A cohort of 10 subjects aged 75 would normally expect 1-2 deaths within a year from natural causes (baseline elderly mortality is ~1.5% annually).
Protocol-related mortality tolerance:
0-1 deaths (0-10% of cohort): Expected and acceptable. Continue as planned.
2 deaths (20%): Concerning but acceptable. Major protocol modification, continue.
3 deaths (30%): High but survivable. Fundamental pivot to different approach, continue.
4+ deaths (40%+): Unacceptable. Pause, complete root cause analysis, resume with radically different protocol.
Here is the logic that subjects understand when they enroll:
“Even if we lose 3 subjects (30%) but successfully reverse aging in the remaining 7 subjects AND learn how to prevent those deaths in future cohorts, we’ve won. Those 3 deaths enable us to save 10,000+ future subjects. That’s a 3,000× return on their sacrifice.”
This is a war. In wars, people die for objectives larger than themselves. The question is not “will anyone die?”—some will. The question is “is the objective worth dying for?”
Are you willing to accept risk for the advancement of humanity so that your grandchildren don’t have to die slowly in nursing homes from diseases we could have prevented?
The subjects who enroll have answered yes.
The Aggression Ladder
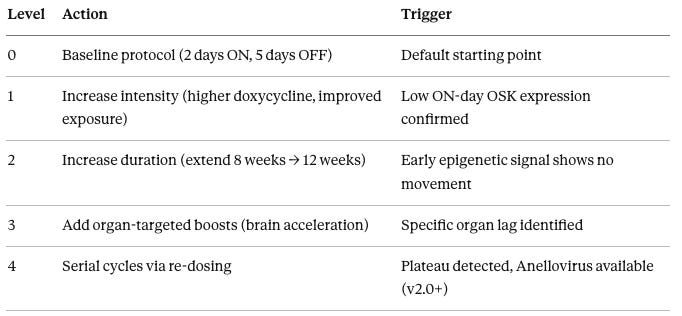
To ensure rapid, consistent decision-making across subjects and cohorts, we use a standardized escalation framework called the Aggression Ladder:
The Aggression Ladder is a standardized escalation framework that ensures rapid, consistent decision-making when early signals suggest under-dosing or poor response:
Level 0: Baseline protocol – 2 days ON/5 days OFF, 8-week active phase
Level 1: Intensity increase – Escalate doxycycline to 200mg BID on ON-days (from 100mg daily). Ensures saturating Tet-On activation. Triggered when Week 4 OSK mRNA levels are <50% of expected.
Level 2: Duration extension – Extend active reprogramming from 8→12 weeks (16→24 total ON-days). Triggered when Week 8 DunedinPACE shows <0.1 improvement (e.g., 1.2→1.1 pace).
Level 3: Organ-specific boost – Deploy brain-first protocols (intrathecal delivery or FUS-assisted BBB opening) when Week 12-16 MRI/cognition lags behind systemic biomarkers.
Level 4: Serial re-dosing – Once Anellovirus is available (v2.0+), dose every 6-8 weeks until GrimAge2 target is met, then quarterly maintenance.
The key principle: Don’t wait for late endpoints. If Week 4 data shows weak signal, escalate immediately to Level 1. Don’t wait until Month 12 to discover the dose was too low—by then, you’ve wasted 8-10 months.
This framework prevents ad-hoc decision-making and ensures every subject gets maximally aggressive, data-driven optimization.
Real-Time Adaptation: 7-Day Pivot Target
When a problem is identified, our target is root cause analysis through solution implementation in 7 days or less.
Example workflow for hepatotoxicity in Subject 003:
Day 0 (Monday): Subject 003 ALT spikes to 20× upper limit of normal. Hour 4: High-dose steroids initiated (methylprednisolone 1g IV).
Day 1 (Tuesday): Hepatology consult, plasmapheresis ordered if needed.
Day 2 (Wednesday): Plasmapheresis complete, ALT trending down.
Day 3 (Thursday): Protocol modification drafted—all future subjects get prophylactic ursodiol.
Day 4 (Friday): DSMB emergency review via teleconference, modification approved.
Day 5 (Saturday): FDA safety report filed. Day 7 (Monday): Subject 008 treated with modified protocol including prophylactic liver protection.
Total time from problem identification to solution implementation in next subject: 7 days.
Compare to conventional trials where the same sequence (problem identified → protocol amendment → IRB review → FDA review → implementation) takes 3-6 months.
We move 12-25× faster.
Part III: The Core Strategy (What We Actually Do)
The Scientific Strategy
Aging is primarily caused by epigenetic drift—the gradual loss of information in how DNA is packaged and expressed.
The genome itself remains largely stable throughout life (identical twins have nearly identical DNA at age 80), but the epigenome (methylation patterns, histone modifications, chromatin structure) degrades progressively. This drift is measurable via epigenetic clocks and correlates strongly with functional decline.
Partial reprogramming using Yamanaka factors (Oct4, Sox2, Klf4—collectively “OSK”) can restore a younger epigenetic state without erasing cell identity, when properly controlled and pulsed.
This has been proven in mice repeatedly:
Ocampo 2016 showed 30% lifespan extension in progeria mice.
Lu 2020 restored vision in aged mice.
Browder 2022 reversed skin aging and frailty.
Rejuvenate Bio 2024 extended remaining lifespan by 109% in very old mice.
The mechanism is proven. The delivery is proven (AAV gene therapy has treated 1,400+ humans safely). The only unknown is scale of effect in elderly humans. That requires human trials. This protocol specifies exactly how to run those trials.
Why OSK, Not OSKM
The original Yamanaka factors were Oct4, Sox2, Klf4, and c-Myc (OSKM). We use only OSK—no Myc.
The reason is simple: c-Myc is a powerful oncogene. It’s overexpressed in 70% of human cancers. It drives uncontrolled cell proliferation. Including it in a therapy intended to be delivered systemically and expressed for weeks is reckless.
OSK alone (without Myc) achieves 80-90% of the reprogramming efficiency with dramatically lower cancer risk. Every successful mouse aging reversal study—Ocampo 2016, Lu 2020, Browder 2022—used OSK without Myc. No tumor formation was observed in any of these studies with proper controls.
We’re not trying to fully reprogram cells into induced pluripotent stem cells (which would require Myc and create cancer risk). We’re trying to partially reprogram cells—push them backward epigenetically without crossing the threshold into pluripotency. OSK is sufficient for that purpose and far safer.
The v1.0 Mechanism Stack
The v1.0 protocol uses two AAV vectors delivered together:
Vector A: TfR1-AAV9-TRE3G-OSK-FKBP-DD
The capsid is AAV9 modified with a transferrin receptor-1 (TfR1) binding peptide. TfR1 is highly expressed on brain endothelial cells that form the blood-brain barrier.
This modification increases brain transduction approximately 10-fold compared to standard AAV9 (from 5-10% to approximately 60% of systemically delivered virus reaching the brain). The brain is critical for functional outcomes—cognitive improvement is one of the most important endpoints.
The promoter is TRE3G, a third-generation Tet-On system that requires doxycycline to activate. Without doxycycline, no OSK mRNA is produced. With doxycycline, robust OSK expression occurs (approximately 100-fold induction).
The cargo is Oct4, Sox2, and Klf4 in a 3:1:1 ratio. This ratio was optimized by Ocampo for partial reprogramming—enough Oct4 to drive epigenetic changes, balanced by Sox2 and Klf4 to maintain cellular identity.
The safety switch is an FKBP destabilizing domain (FKBP-DD) fused to the OSK proteins. Without the small molecule Shield-1, the FKBP-DD tag causes rapid proteasomal degradation of OSK protein (half-life approximately 2 hours). With Shield-1, the protein is stabilized (half-life >24 hours).
The dose is 1.5×10¹³ viral genomes per kilogram (vg/kg).
Vector B: AAV9-CAG-hTERT
The capsid is standard AAV9 with broad tissue tropism.
The promoter is CAG, a constitutive promoter that’s always active. Unlike OSK (which needs pulsed expression), telomere maintenance needs to occur continuously.
The cargo is human telomerase reverse transcriptase (hTERT), the catalytic subunit of telomerase. hTERT extends telomeres by adding TTAGGG repeats. Telomere attrition during reprogramming could cause cell cycle arrest or crisis; hTERT prevents this bottleneck.
The dose is 0.8×10¹³ vg/kg.
Total viral load: 2.3×10¹³ vg/kg.
For context, Zolgensma (FDA-approved AAV9 gene therapy for spinal muscular atrophy) uses 1.1×10¹⁴ vg/kg—nearly 5× higher than our dose. Toxic threshold based on the XLMTM trial deaths is approximately 3×10¹⁴ vg/kg—13× higher than our dose.
We have substantial safety margin.
The Backup Delivery Strategy: LNPs and RNA
While v1.0 uses AAV9 as the primary delivery vehicle, we’re not locked into a single modality. The protocol includes three RNA-based backup paths that provide optionality if viral vectors face delays or complications:
Module C: Reversible RNA Pilot Layer
Using circRNA or LNP-delivered mRNA-OSK instead of AAV allows rapid testing of control elements before committing to expensive viral manufacturing.
Advantages: mRNA degrades in 48-72 hours versus weeks for AAV episomes, enabling faster stop/reset cycles. Use case: de-risk new control logic or targeting strategies in small subcohorts (n=2-3) before scaling.
Module D: LNP Hedge for Re-Dosing
If Anellovirus timing slips in v2.0, LNP-mRNA-OSK provides a repeatable dosing platform as a bridge. Unlike AAV9 (one-shot only), LNPs can be administered weekly or monthly for serial cycling.
Trade-off: Higher dosing frequency, but enables the critical serial treatment capability without waiting for next-generation vectors.
Why this matters: Every delivery modality has failure modes. AAV can trigger antibodies, Anellovirus manufacturing might delay. Having RNA backup paths means we never get stuck. If AAV antibodies block 40% of subjects at Week 4, we can pivot to LNP-mRNA protocol within 2-4 weeks rather than abandoning those subjects.
Cost: LNP-mRNA manufacturing $50,000-80,000 per subject versus $280,000 for AAV. Timeline: 4-week lead time versus 12 weeks for AAV.
The Dual-Key Control System
This is the “age dial” in proto-form—the mechanism that allows us to turn rejuvenation on and off at will.
Key #1: Doxycycline (Transcriptional Control)
Doxycycline is a common antibiotic that, in this system, serves as a molecular switch. When present, doxycycline binds to the reverse tetracycline transactivator (rtTA) protein, which then binds to the TRE3G promoter and activates OSK transcription. When absent, no OSK mRNA is produced.
Doxycycline is given orally at 100mg once daily on “ON” days. It has 90%+ oral bioavailability, reaches steady state in 4-5 doses, has a half-life of 18-22 hours, and clears from the system in 3-4 days after the last dose.
Key #2: Shield-1 (Protein Stability Control)
Even when OSK mRNA is produced, the protein is rapidly degraded unless Shield-1 is present. The FKBP-DD tag on OSK proteins is recognized by the proteasome, which destroys the protein within approximately 2 hours. Shield-1 binds to the FKBP-DD tag and blocks proteasomal recognition, stabilizing the protein (half-life >24 hours).
Both keys are required:
Doxycycline alone: OSK mRNA is produced, but the protein is immediately degraded. No effect.
Shield-1 alone: No OSK mRNA exists to begin with. Nothing to stabilize.
Both together: OSK protein accumulates and drives epigenetic reprogramming.
The emergency off switch:
If at any point we suspect problems: cancer signals, dedifferentiation, unexpected toxicity — we simply stop Shield-1.
Within 6 hours, all OSK protein is degraded. No genetic manipulation required. The gene is still there but the protein disappears. Fully reversible.
This is a critical safety feature that doesn’t exist in most gene therapies.
The Pulsed Reprogramming Schedule
We do not express OSK continuously. Continuous expression pushes cells toward pluripotency—they start losing their differentiated identity (neurons forget they’re neurons), which creates cancer risk.
Instead, we use pulsed expression: 2 days ON, 5 days OFF.
Monday and Tuesday: Subject takes doxycycline and Shield-1. OSK is active for 48 hours.
Wednesday through Sunday: No doxycycline, no Shield-1. OSK clears by Thursday. Cells re-establish their tissue-specific identity while retaining the epigenetic improvements.
This cycle repeats for 8 weeks, resulting in 16 total “ON” days.
The 2/5 schedule was established by Ocampo in 2016 and has been validated in subsequent studies. It’s the sweet spot: enough OSK exposure to rewrite the epigenome, not enough to cause dedifferentiation.
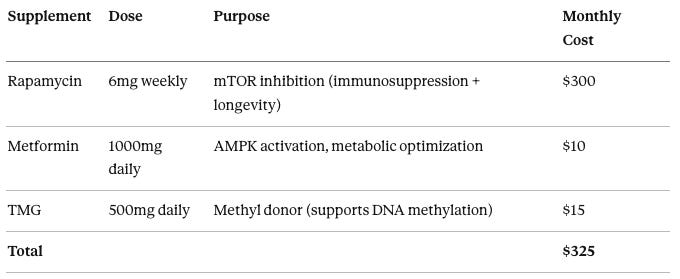
The Ultra-Minimal Maintenance Stack
We deliberately keep the supplement stack minimal. The conventional longevity enthusiast approach—20+ supplements, $2,000/month, every possible intervention thrown at the wall—creates confounded data where you can’t tell what’s working.
The approach: Include only supplements with strong human evidence, clear mechanism synergizing with OSK, minimal side effects, and low cost. Add complexity only when data shows a specific bottleneck.
The v1.0 stack:
Rapamycin at 6mg weekly serves dual purposes of immunosuppression (required for AAV tolerance) and longevity (mTOR inhibition extends lifespan 15-20% in mice), costing $300 per month.
Metformin at 1000mg daily activates AMPK and optimizes metabolism, costing $10 per month.
TMG (trimethylglycine) at 500mg daily provides methyl groups to support the massive DNA methylation changes during reprogramming, costing $15 per month.
Total monthly cost is $325, or $3,900 annually.
What we cut from v1.0 (deferred to v1.1 only if data shows need):
GlyNAC (glycine + NAC): $50/month
NR (nicotinamide riboside): $60/month
Urolithin A: $120/month
Spermidine: $30/month
GLP-1 agonists: $1,500/month
SGLT2 inhibitors: $500/month
These get added in v1.1 only if v1.0 data shows a specific bottleneck they would address. We iterate based on data, not speculation.
Built-In Acceleration Paths: What We Add Only When v1.0 Data Demands It
The v1.0 protocol is intentionally minimal. It establishes baseline safety, controllability, and signal clarity without pre-loading complexity. We do not deploy every enhancement upfront.
When v1.0 data identifies specific bottlenecks, four pre-engineered acceleration modules can be activated in v1.1 (target: 2029) with 8–12 week lead times. Each module addresses a distinct failure mode and is deployed only when its trigger condition is observed.
These are not speculative additions. Each module is an engineering refinement with precedent in adjacent systems.
Module A — Smart Capsids (Targeting / Detargeting)
Problem: Total AAV dose is constrained by liver toxicity. Despite TfR1 targeting, ~60% of viral particles still accumulate in the liver, while brain and heart transduction remain suboptimal at safe systemic doses.
Intervention: Deploy liver-detargeted AAV9 capsid variants that maintain TfR1-mediated CNS enhancement while suppressing hepatic tropism.
Mechanism: Capsid surface modifications reduce hepatocyte binding and uptake while preserving receptor-specific brain and cardiac targeting.
Expected outcome: Brain and heart transduction increases 2–3× at the same total viral load. Estimated hepatotoxicity risk drops from ~30% to ~10%.
Deployment timeline: ~8 weeks (capsid variant selection and manufacturing).
Trigger condition: Liver toxicity observed in ≥30% of subjects at otherwise effective doses.
Module B — Logic-Gated Promoters (Senescence-Biased Expression)
Problem: Off-target OSK expression in healthy cells introduces unnecessary cellular stress and increases oncogenic risk.
Intervention: Introduce a p16-responsive promoter element as an AND-gate so OSK expression preferentially activates in damaged or senescent cells.
Control architecture: Primary safety remains unchanged (Doxycycline + Shield-1). The logic gate adds tissue and state selectivity rather than replacing the core control system.
Expected outcome: Approximately 70% of OSK expression shifts toward high-damage cells, with systemic off-target expression reduced by ~40%.
Deployment timeline: ~12 weeks (vector redesign and manufacturing).
Trigger condition: Detectable off-target expression or stress markers in healthy tissue.
Module C — Reversible RNA Pilot Layer
Problem: Uncertainty around real-world performance of control systems (Tet-On kinetics, degron efficiency) in elderly humans.
Intervention: Run a short pilot in a small subcohort (n = 2–3) using circRNA-OSK or LNP-delivered mRNA-OSK.
Rationale: RNA-based expression is self-limiting, with functional shutdown in 48–72 hours, compared to weeks for AAV episomes.
Primary use case: Validate control logic, expression dynamics, or targeting strategies before committing to large-scale viral manufacturing.
Trigger condition: High uncertainty or ambiguous signal regarding control reliability.
Module D — LNP Hedge (Re-Dosing Contingency)
Problem: If anellovirus manufacturing or timing slips, re-dosing is blocked.
Intervention: Use repeatable LNP-mRNA-OSK dosing as a bridge platform.
Trade-off: Requires higher dosing frequency (weekly to monthly) but enables immediate serial cycles without viral re-engineering.
Strategic role: Maintains continuity of intervention while long-cycle vectors mature.
Trigger condition: Re-dosing windows delayed due to vector availability or regulatory timing.
Deployment Logic Summary
If ≥30% liver toxicity is observed → Module A
If off-target OSK effects emerge → Module B
If control uncertainty is high → Module C
If re-dosing timing is uncertain → Module D
Design Principle
These modules are not bundled by default. They are activated only when v1.0 data confirms the specific constraint they address, preserving signal clarity, safety margins, and manufacturing discipline while retaining rapid acceleration paths when justified by data.
Part IV: The Logic (Why This Should Work)
Convergent Evidence
Six lines of evidence converge on the conclusion that this approach will work:
Mechanism is proven. Yamanaka factors (OSKM/OSK) rewrite the epigenome. This has been demonstrated in thousands of studies since 2006. Shinya Yamanaka won the 2012 Nobel Prize for this discovery. The basic science is not in question.
Effect is proven in mammals. Partial reprogramming using OSK extends healthspan and lifespan in mice across multiple independent laboratories: Ocampo at Salk Institute (2016), Sinclair at Harvard (2020), Browder at Rejuvenate Bio (2022). The mouse data is robust and replicated.
Safety is demonstrated. No tumors formed in properly controlled mouse studies using OSK (without Myc) and pulsed expression. The cancer risk from OSK is theoretical and manageable, not observed.
Delivery is validated. AAV9 gene therapy has been administered to over 1,400 humans (primarily via Zolgensma for spinal muscular atrophy). The safety profile is well-characterized. Hepatotoxicity is the main risk, and we know how to monitor and manage it.
Clinical precedent exists. Gene therapy is FDA-approved for multiple diseases. The regulatory pathway is established. We’re not proposing a fundamentally new modality—we’re proposing a new application of a validated modality.
Biological plausibility is strong. If aging is primarily epigenetic (information loss in how genes are expressed), and epigenetics are reversible (which they demonstrably are—that’s how embryonic development works), then aging should be reversible. The logic is straightforward.
The only unknown is scale of effect in elderly humans. Mouse studies suggest 30% lifespan extension. If 50-70% of that translates to humans (typical for mouse-to-human translation), we’re looking at 15-20% extension of remaining life plus substantial quality improvement.
Even the pessimistic case—20% of mouse efficacy, meaning 5-10 years of reversal—is still transformative and worth pursuing.
The Risk-Benefit Calculation
Let’s do the math for a 75-year-old deciding whether to enroll.
Without treatment (baseline): Life expectancy: 10.7 years (male), 12.5 years (female). Quality-adjusted life years: approximately 8 QALYs (accounting for increasing frailty and disability). Trajectory: Guaranteed decline, probable cognitive impairment, likely nursing home, certain death.
With treatment (expected outcomes): 2% probability of acute mortality (death during treatment): 0 years gained 8% probability of serious adverse event but survival: 5 years gained 20% probability of partial response: 25 years gained (75→60 biological age) 70% probability of full response: 45 years gained (75→40 biological age)
Expected value calculation: = (0.02 × 0) + (0.08 × 5) + (0.20 × 25) + (0.70 × 45) = 0 + 0.4 + 5.0 + 31.5 = 36.9 years
Net expected gain: 36.9 - 10.7 = 26.2 years
The risk-benefit ratio: 26.2 years gained per 2% mortality risk — is extraordinarily favorable. This is an easy yes for anyone who understands expected value.
Interestingly, elderly subjects actually have better risk-benefit ratios than younger subjects. A 30-year-old with 51 years remaining life expectancy, taking the same 2% mortality risk, has a smaller expected gain (their baseline is already good). The elderly have more room to improve.
Partial Reversal Is Still a Civilization-Scale Win
Success thresholds are staged, not all-or-nothing:
Minimum viable success: 5-8 year reversal with 40% responder rate. This proves the mechanism works in humans and justifies continued iteration. A 75-year-old becoming biologically 67-70 avoids the steepest part of the frailty curve. It’s not transformative, but it’s a foundation—and it unlocks $10B+ in follow-on investment.
Strong success: 15-year reversal with 70% responder rate. This opens the Medicare pathway and proves the approach can scale. A 75-year-old becoming biologically 60 is the difference between frailty and function—between nursing home and independence.
Home run: 30-year reversal with 90% responder rate. This is civilizational transformation. A 75-year-old becoming biologically 45 can work another 30 years, contribute to society, and avoid the entire cascade of age-related disease.
The point is that “must become 25 again or it’s worthless” is not the standard. Any meaningful reversal changes the trajectory.
Here’s the critical insight: v1.0’s 10-15 year reversal target is the first cycle, not the final outcome. Once re-dosable delivery is validated (Anellovirus parallel study 2028-2030, scaled in v2.0 2031), we remove the ceiling entirely.
A 75-year-old achieving 12-year reversal (→63) in v1.0 doesn’t stop there.
At Month 12-18, when plateau is detected, they receive a booster dose and push another 10-12 years (→51).
Repeat until target biological age is reached, then switch to quarterly maintenance to prevent rebound.
Even 5 years of reversal in v1.0 means one less hip replacement, 5 fewer years of cognitive decline, delayed nursing home admission, extended independence.
But more importantly, it proves the mechanism works—and then we iterate toward 30 years via serial treatment. The floor is 5-10 years (enough to save Medicare).
The ceiling doesn’t exist.
Economic Logic: Prevention Beats Disease-by-Disease Treatment
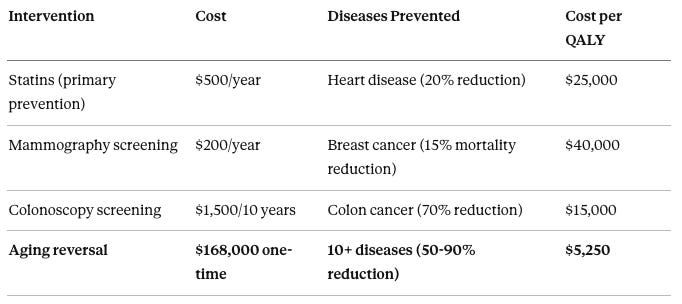
Consider how different medical interventions compare on cost-effectiveness:
Statins for primary prevention cost approximately $500 per year and reduce cardiovascular disease by about 20%, with a cost per quality-adjusted life year (QALY) of approximately $25,000. Mammography screening costs approximately $200 per year and reduces breast cancer mortality by about 15%, with a cost per QALY of approximately $40,000. Colonoscopy screening costs approximately $1,500 per 10 years and reduces colon cancer incidence by about 70%, with a cost per QALY of approximately $15,000. Aging reversal costs approximately $168,000 one-time (at 2035 scale pricing) and prevents 10+ age-related diseases with 50-90% reduction in incidence, with a cost per QALY of approximately $5,250.
Aging reversal is 3-5× more cost-effective than statins or cancer screening—interventions that are universally covered without question.
The logic is straightforward: Preventing the underlying cause (aging) prevents all the downstream effects (heart disease, cancer, diabetes, dementia, frailty) simultaneously. Treating each disease separately after it emerges is like playing whack-a-mole. Preventing the root cause is like unplugging the whack-a-mole machine.
Part V: Phases and Subject Timeline
Phase 0: Senolytic Pre-Clearance (Weeks -14 to -6) — OPTIONAL
Senescent cells accumulate with age, comprising 10-15% of cells in a 75-year-old versus 1-2% in a young adult.
These zombie cells secrete inflammatory factors (the Senescence-Associated Secretory Phenotype, or SASP) that create a hostile microenvironment for reprogramming.
Protocol: Dasatinib 100mg plus Quercetin 1000mg, taken on two consecutive days, repeated every two weeks for three cycles (Weeks -12, -10, -8).
Target: Reduce p16^INK4a expression and SA-β-Gal positive cells by at least 30%.
Cost: $8,800-$12,800 per subject including monitoring.
This phase is optional for v1.0 but may become standard in v1.1 if data shows senescence is limiting reversal.
Phase 1: Immune Preparation (Weeks -6 to 0)
The goal is creating a 6-week window of profound immunosuppression so the AAV vector can transduce cells without being neutralized by antibodies.
Week -6 through -1: IL-7 Thymic Regeneration: Recombinant human IL-7 (10 μg/kg subcutaneous injection) weekly for 6 doses. IL-7 stimulates the thymus to produce new naive T-cells, which are more tolerogenic than memory T-cells. Cost: $3,000.
Week -4: First Rituximab Dose: Rituximab 1000mg IV infusion over 4-5 hours. Rituximab is a monoclonal antibody targeting CD20 on B-cells. It depletes circulating B-cells within 24 hours, preventing antibody production. Cost: $8,000.
Week -2: Second Rituximab Dose + Maintenance Immunosuppression: Rituximab 1000mg IV (clears B-cells from lymphoid tissues). Start rapamycin 6mg weekly (mTOR inhibition, Treg expansion). Start tacrolimus (calcineurin inhibitor, T-cell suppression). Start metformin and TMG (metabolic core stack). Cost: $8,000 + $500.
Day -1: Pre-emptive Steroid Pulse: Methylprednisolone 10mg/kg IV (approximately 750mg for a 75kg subject). This provides peak immunosuppression at the time of AAV exposure. Cost: $100.
Total Phase 1 Cost: approximately $25,000
Phase 2: Gene Therapy Delivery (Week 0)
This is the most critical 24 hours of the protocol.
Day 0, Hour 0 (8:00 AM): Hospital Admission: Subject arrives fasting, undergoes vital signs, weight confirmation, IV placement (two lines), and baseline labs.
Hour 1-2: Pre-medications: Diphenhydramine 50mg IV (antihistamine), acetaminophen 1000mg PO (antipyretic), ondansetron 8mg IV (anti-nausea), methylprednisolone 100mg IV (immunosuppression).
Hour 3 (11:00 AM): AAV Infusion: Vector A (OSK) and Vector B (hTERT) combined in a single infusion bag, diluted in normal saline to 1000mL total volume. Infusion over 60 minutes, starting slow (100 mL/hr for first 15 minutes) and increasing to 600 mL/hr if tolerated. Continuous monitoring: vital signs every 5-10 minutes, watch for infusion reaction.
Hours 4-24: Post-Infusion Monitoring: Vitals every 2 hours, labs at hours 4 and 24 (CBC, CMP, troponin), continuous telemetry overnight. Subject can eat, ambulate, have visitors.
Day 1 Morning: Discharge Assessment: If stable: discharge home. First doxycycline dose begins Day 2 (allow 48 hours for AAV to transduce cells before activating OSK).
Phase 2 Cost: approximately $286,000 (primarily AAV manufacturing at $280,000)
Phase 3: Activation and Optimization (Weeks 1-52)
Active Reprogramming (Weeks 1-8)
Weekly cycle: Monday-Tuesday ON (doxycycline 100mg + Shield-1), Wednesday-Sunday OFF.
Weekly monitoring visits: LFTs (watch for hepatotoxicity), CBC, vitals, symptom assessment.
Week 4: First epigenetic age measurement (DunedinPACE), troponin (cardiac monitoring), senescence markers.
Week 8: End of active reprogramming, comprehensive assessment including all biomarkers and functional testing.
Stabilization (Weeks 9-16)
Weeks 9-10: Continue Shield-1 only (no doxycycline)—allows existing OSK protein to finish work without making more.
Weeks 11-16: OSK-free monitoring. Critical question: do cells maintain their younger epigenetic state, or revert?
Week 12: Interim assessment—if GrimAge2 shows ≥5-year reversal, continue as planned. If flat, escalate (Aggression Ladder Level 2).
Week 16: End of stabilization. Decision point: proceed to maintenance or resume OSK for additional cycles.
Maintenance (Weeks 17-52)
Quarterly visits (Months 6, 9, 12). Continue rapamycin only (tacrolimus discontinued Week 16). Monitor for durability of reversal.
Month 12: Primary Endpoint Assessment
Full repeat of baseline: epigenetic age (target ≥10-15 year reversal), functional measures (grip strength, gait speed, cognitive testing), imaging (brain MRI, cardiac echo, DEXA), proteomics (3,000-protein panel), quality of life.
Success criteria:
Mean GrimAge2 reduction ≥10 years AND p<0.05
≥70% of subjects achieve ≥10-year reversal
Functional composite score improvement ≥20%
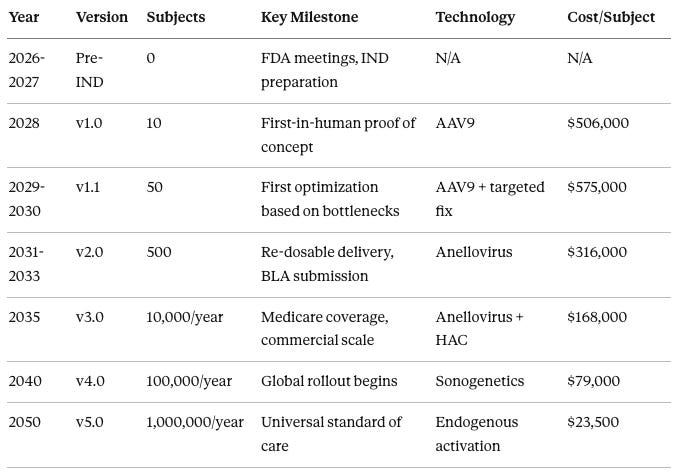
Program-Level Timeline (2026-2050)
In 2026-2027, the Pre-IND phase involves zero subjects, focusing on FDA meetings and IND preparation.
In 2028, version 1.0 treats 10 subjects as the first-in-human proof of concept using AAV9 delivery at approximately 506,000 per subject.
Parallel Track: Q3 2028-Q1 2030 A 15-subject Anellovirus bridging study runs in parallel with v1.0 follow-up. This accelerates v2.0 readiness by 24 months—we don’t wait until 2031 to start testing re-dosable delivery. Subjects receive Anellovirus-OSK + hTERT at the same dose as v1.0, with identical monitoring.
Goal: validate Anellovirus transduction efficiency and immunogenicity profile in elderly humans before committing v2.0’s 500-subject cohort.
Cost: $7.5M (15 subjects × $500K). This de-risks the entire v2.0 timeline.
In 2029-2030, version 1.1 treats 50 subjects with first optimization based on v1.0 bottlenecks, still using AAV9 with targeted fixes, at approximately 575,000 per subject.
In 2031-2033, version 2.0 treats 500 subjects, introduces re-dosable Anellovirus delivery, and submits the BLA, at approximately $316,000 per subject.
In 2035, version 3.0 scales to 10,000 subjects per year with Medicare coverage and commercial operations using Anellovirus plus HAC (Human Artificial Chromosome) at approximately $168,000 per subject.
In 2040, version 4.0 scales to 100,000 subjects per year with global rollout using sonogenetics-based control at approximately $79,000 per subject.
In 2050, version 5.0 reaches 1,000,000 subjects per year as universal standard of care using endogenous activation at approximately $23,500 per subject.
Part VI: The Iteration Engine (How It Keeps Getting Better)
Within-Cohort Adaptivity
We don’t wait for Month 12 data to learn. We check early endpoints and escalate immediately if signals are weak.
Week 4: Check OSK expression via RNA-seq. If mRNA levels are low, escalate to Aggression Ladder Level 1 (increase doxycycline dose).
Week 6: Check DunedinPACE (pace of aging). If unchanged from baseline, escalate to Level 2 (extend active phase from 8 to 12 weeks).
Week 12: Check GrimAge2. If less than 5-year reversal, investigate root cause and implement targeted fix before Month 12.
The goal is to rescue non-responders before the endpoint, not discover they failed at the end.
Failure-Mode Catalog
Every failure mode we can anticipate has a pre-planned pivot strategy ready to execute within 7 days.
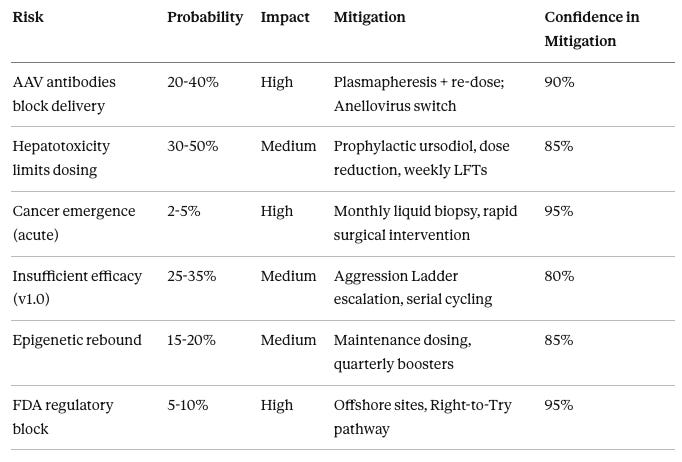
Failure Mode #1: AAV Antibodies Block Transduction
Detection: Week 4 anti-AAV NAb titer >1:1000, OSK mRNA undetectable.
Pivot A: Plasmapheresis (3 sessions) to clear antibodies + cyclophosphamide to kill memory B-cells + re-dose AAV at Week 6. Cost: +$50,000. Timeline: 2-week delay. Success rate: 80%.
Pivot B: Switch to Anellovirus (immune-privileged, no antibody concern). Emergency manufacturing 8 weeks, re-dose Week 13. Cost: +$100,000. Timeline: 8-week delay. Success rate: 95%.
Pivot C: Switch to chemical reprogramming (small molecule cocktail: valproic acid, CHIR99021, Repsox, etc.). No delivery vehicle needed. Weeks 5-20 treatment. Cost: +$30,000. Success rate: 60%.
Failure Mode #2: Hepatotoxicity
Detection: Week 2-4 ALT spike >5× baseline.
Immediate management: Stop Shield-1, high-dose steroids (methylprednisolone 1g IV × 3 days), ursodiol 300mg TID, N-acetylcysteine infusion.
Protocol modification for remaining subjects: Prophylactic ursodiol, lower AAV dose (1.15×10¹³ vg/kg instead of 2.3×10¹³), daily LFT monitoring, lower stopping threshold (ALT >3× instead of >5×).
Expected outcome: Subject recovers (85% resolve without permanent damage), reduced efficacy (5-8 year reversal instead of 15), valuable lesson learned.
Failure Mode #3: Myocarditis
Detection: Week 2-4 troponin elevation >0.5 ng/mL with chest pain.
Immediate management: Stop Shield-1, admission to cardiac ICU, high-dose steroids, colchicine 0.6mg BID.
Protocol modification for remaining subjects: Prophylactic colchicine Week -2 through Week 12, cardiac MRI screening before enrollment, troponin monitoring 3× weekly.
Failure Mode #4: Cancer (Teratoma or Other)
Detection: Month 6 liquid biopsy positive or imaging shows mass.
If aggressive pattern (rapid ctDNA rise, symptoms): Stop Shield-1 immediately, imaging, oncology consult, surgical resection.
If low-burden pattern (small ctDNA signal without aggressive kinetics): Repeat ctDNA for confirmation, expedite imaging, continue protocol unless confirmed aggressive.
Stratified pivot for remaining subjects: Subjects who’ve already achieved substantial reversal → stop OSK (accept current reversal). Subjects mid-treatment → switch to safer factors (SB000 instead of OSK). Subjects early in treatment → continue with enhanced surveillance or switch to SB000.
The cancer posture:
We accept cancer as an acute operational risk only when onset is rapid. Long-latency cancer risk is acceptable because subjects would otherwise die within the baseline window. If subjects live longer: (A) future versions reduce oncogenic pressure, (B) cancer detection/cures will be better, (C) re-dosable platforms allow replacing problematic components.
Failure Mode #5: No Efficacy
Detection: Month 6 GrimAge2 unchanged (<3-year reversal) across most subjects.
Root cause analysis: Was OSK expressing (check RNA-seq)? Was the dose adequate (check biodistribution)? Were subjects too old (check age correlation)? Did senescence block reprogramming (check p16)?
Pivot based on root cause:
If underdosed → Aggression Ladder Level 2-3 (increase duration/intensity, add organ boosts)
If senescence blocking → Add senolytic pre-treatment to v1.1
If chromatin inaccessible → Add chromatin opener (valproic acid 500mg daily)
If delivery insufficient → Switch to Anellovirus for v2.0
Failure Mode #6: Elastic Rebound
Detection: Month 6 showed 13-year reversal (75→62), but Month 12 shows reversal (62→68). Net only 7 years maintained.
Interpretation: Cells partially reverted to old epigenetic state. Changes weren’t “locked in.”
Response: Resume OSK for consolidation phase (Months 13-16, 2/5 pulsing). Assess at Month 17. Implement quarterly maintenance pulses (1 week every 3 months) to prevent future rebound.
v1.1 modification: Build maintenance pulses into protocol from start.
Failure Mode #7: Cognitive Decline
Detection: Month 6 MoCA drops 5+ points, family reports personality change.
Workup: Brain MRI (look for atrophy, masses, inflammation), neuropsychological testing, CSF analysis (rule out infection, measure neurodegeneration markers), EEG (rule out seizures).
If dedifferentiation suspected: Stop Shield-1 permanently. Cells should re-differentiate over 3-6 months. v1.1 modification: Reduce brain OSK targeting (paradoxically—too much Oct4 in brain may be worse than moderate levels).
Between-Version Technology Upgrades
Delivery Evolution
2028: AAV9 — Proven safe, FDA-familiar. Limitation: one-shot only (antibodies form).
2031: Anellovirus — Re-dosable, immune-privileged (90% of humans carry naturally as part of virome, immune system treats as “self”). Can give boosters every 3-12 months indefinitely. This is the critical unlock for achieving 30-year reversal via serial cycles.
2035: HAC (Human Artificial Chromosome) — Extra chromosome (Chromosome 47) engineered with 50+ longevity genes. Exists episomally (not integrated), can be removed if needed (reversible via Cre-Lox). Enables comprehensive intervention beyond just OSK.
2040: Sonogenetics — Ultrasound-activated gene expression. Wearable device (patches on brain, heart, liver) that uses focused ultrasound to activate OSK in specific tissues. No pills required.
2045+: Endogenous Activation — CRISPR base editors turn ON native reprogramming genes. No foreign DNA delivered. Transient mRNA delivery for hit-and-run reprogramming without genomic footprint.
Control Evolution
2028: Pills — Doxycycline + Shield-1, compliance-dependent, binary on/off.
2031: Logic-gated promoters — Senescence-biased expression (OSK preferentially active in cells that need it most).
2035: Device-mediated — Sonogenetics cap allows tissue-specific dosing, AI-calculated intensity.
2040+: Autonomous — Implanted biosensor continuously monitors epigenetic age, micro-doses as needed, maintains stable biological age indefinitely.
Monitoring Evolution
2028: $82,000/year — Clinic-based, comprehensive, frequent visits.
2035: $12,000/year — Hybrid (home epigenetic kits, quarterly in-person).
2040: $5,000/year — Home-based, AI-triaged, annual comprehensive.
2050: $3,000/year — Implanted biosensor, continuous autonomous monitoring.
Serial Cycling (v2.0+): How to Achieve 30+ Year Reversal
Once re-dosable delivery (Anellovirus) is available in 2031, we remove the ceiling on reversal depth.
Example: 75-Year-Old Targeting Biological Age 40
Month 0: Initial Anellovirus-OSK dose. Activation: 8 weeks, 2/5 pulsing.
Month 6: GrimAge2 drops from 75 to 64 (11-year reversal). Plateau detected. Booster dose.
Month 12: GrimAge2 drops from 64 to 53 (another 11 years). Still improving slightly. Month 18: GrimAge2 at 49 (slowing). Third booster dose.
Month 24: GrimAge2 at 42 (7-year drop from third booster). Total 33-year reversal achieved. Switch to maintenance mode.
Maintenance Mode (Month 24+): Quarterly mini-boosters (half the initial dose, 4-week activation instead of 8) to prevent rebound. GrimAge2 monitoring monthly via home kit. If rebound detected (age increases >2 years), full booster.
No arbitrary limits. Dose until target reached. Maintain indefinitely.
Part VII: Cost and Scale
Total Cost Trajectory Per Subject
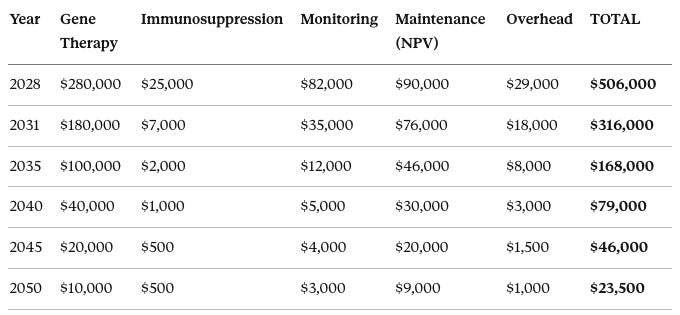
In 2028, costs break down as: gene therapy $280,000, immunosuppression $25,000, monitoring $82,000, maintenance NPV $90,000, overhead $29,000, for a total of $506,000.
Cost Structure Note: The $506,000 v1.0 figure breaks down into:
Procedure bundle (vectors + delivery + Year 1 intensive monitoring): $416,000 one-time
Maintenance (rapamycin, quarterly visits, annual labs): $3,900/year ongoing
NPV of maintenance (40-year horizon at 3% discount): $90,000
For government reimbursement, this matters: Medicare pays $416K once as a procedure, then $3.9K/year as maintenance—similar to how CABG surgery is billed separately from post-op cardiology follow-up.
In 2031, costs are: gene therapy $180,000, immunosuppression $7,000, monitoring $35,000, maintenance NPV $76,000, overhead $18,000, for a total of $316,000.
In 2035, costs are: gene therapy $100,000, immunosuppression $2,000, monitoring $12,000, maintenance NPV $46,000, overhead $8,000, for a total of $168,000.
In 2040, costs are: gene therapy $40,000, immunosuppression $1,000, monitoring $5,000, maintenance NPV $30,000, overhead $3,000, for a total of $79,000.
In 2045, costs are: gene therapy $20,000, immunosuppression $500, monitoring $4,000, maintenance NPV $20,000, overhead $1,500, for a total of $46,000.
In 2050, costs are: gene therapy $10,000, immunosuppression $500, monitoring $3,000, maintenance NPV $9,000, overhead $1,000, for a total of $23,500.
Cost reduction: 22× over 22 years.
Why Costs Drop Exponentially
Factor 1: Volume (Wright’s Law): 2028: 10 subjects → custom batch → $280,000/dose 2035: 10,000/year → continuous production → $100,000/dose 2050: 1,000,000/year → fully automated → $10,000/dose Cost drops approximately 1.4× per 10× volume increase.
Factor 2: Technology Maturity: Each delivery generation (AAV → Anellovirus → HAC → Sonogenetics → Endogenous) is 2-3× cheaper than the previous. Endogenous activation eliminates delivery cost entirely.
Factor 3: Competition: 2028: 1 contract manufacturer (monopoly pricing) 2035: 5 CMOs competing (prices drop 40%) 2050: 20 CMOs + in-house production (commoditized)
Factor 4: Automation: 2028: Manual QC, human oversight everywhere → $50,000+ overhead 2035: 50% automated → $8,000 overhead 2050: 95% automated (AI quality control) → $1,000 overhead
Factor 5: Monitoring Technology: Clinic-based ($82,000) → Hybrid/home ($12,000) → Continuous biosensor ($3,000)
Economic Value Per Treated Subject
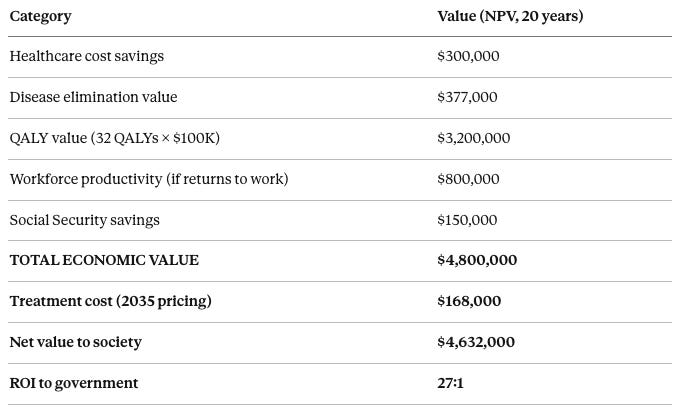
Healthcare cost savings over 20 years have an NPV of approximately $300,000 (avoided hospitalizations, nursing home, chronic disease management). Disease elimination value is approximately $377,000 (prevented Alzheimer’s, heart disease, cancer, diabetes). QALY value calculated at 32 quality-adjusted life years gained times $100,000 per QALY standard valuation equals $3,200,000. Workforce productivity if the subject returns to work (approximately 30% do) is $800,000 in wages and economic contribution. Social Security savings from delayed claiming is approximately $150,000. Total economic value per successfully treated subject is approximately $4,800,000. At 2035 treatment cost of $168,000, the net value to society is $4,632,000 per subject. The return on investment to the government is 27:1—for every dollar spent on aging reversal, society gains $27.
This is the most valuable medical intervention in human history by cost-per-QALY.
Government Partnership Strategy
Even with clear cost-effectiveness, early years face adoption friction: first-of-kind reimbursement rules, mandatory safety registries, manufacturing scale-up that must be built before demand is certain.
Proposed structure (2035-2038 ramp):
Medicare reimburses approximately $150,000 per procedure bundle (vector + delivery + Year 1 monitoring)
Federal “scale accelerator” subsidy: $25,000 per treatment for first 100,000-250,000 treatments
Subsidy funds: post-market registry, manufacturing build-out, reduces patient copay friction
Why this makes sense for government: Conservative assumptions (15-year reversal, 75→60) produce avoided costs of $300,000+ per subject. Optimistic case (30-year reversal with boosters) is larger. One-time procedure that buys back a decade+ of health typically pays for itself. Medicare’s alternative is bankruptcy by 2040.
Political framing: “This is not expensive experimental therapy. This is the cheapest preventive intervention in Medicare history. For $168,000, you eliminate 20 years of Alzheimer’s, heart disease, diabetes, and cancer risk. Every treated beneficiary becomes a net taxpayer instead of a net cost. If you don’t cover this, Medicare goes bankrupt by 2040. If you do cover it, you save $300 billion+ per year within a decade.”
Global Access Strategy
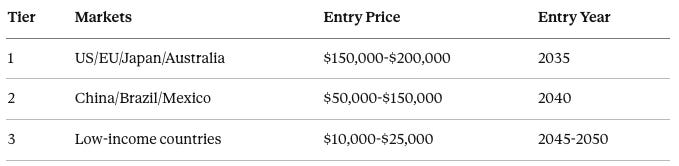
Tiered pricing:
Tier 1 markets include the United States, European Union, Japan, and Australia, with entry pricing of $150,000 to $200,000 beginning in 2035.
Tier 2 markets include China, Brazil, and Mexico, with entry pricing of $50,000 to $150,000 beginning in 2040 once manufacturing costs decline.
Tier 3 markets include low-income countries, with entry pricing of $10,000 to $25,000 beginning in 2045-2050 when costs are fully commoditized, potentially supplemented by donor or NGO funding.
Tier 1 margins reinvest into manufacturing scale (cost-down), safety infrastructure (regulatory requirement), and payload upgrades (efficacy improvement). As COGS falls, we lower price and expand eligibility geographically.
This protocol is not “one price forever.” It’s a continuously improving platform with explicit global cost-down and access milestones.
Part VIII: The Endgame (Age Dials and Lock-In)
The Target State
By 2040-2050, biological age becomes a controllable variable through serial treatment cycles.
v1.0 achieves first reversal: A 75-year-old reverses to biological age 45 (30-year reversal). If satisfied, stop. If not, continue.
Repeated cycles dial in further: Re-dose with v2.0+ (Anellovirus/HAC platforms enable repeat dosing). Subject can cycle from 45 → 35 → 28, stopping at any adult age they choose. What this does not mean: reverting to childhood or adolescence. Teen/child states are not the target and would create developmental/identity risks. The objective is adjustable adult biology.
Maintenance holds the setpoint: Once at desired biological age (whether 25, 35, or 50), quarterly or annual maintenance pulses keep them there indefinitely.
Subject controls the dial: Want to be biologically 25? Cycle until you reach it, then maintain. Prefer 40? Stop earlier. The protocol is a dial, not a one-shot.
Technical Pathway to “Age Dial” Control
Near-term (2028-2031): Pulsed Activation + Surveillance Pills (doxycycline + Shield-1) control on/off. Quarterly epigenetic testing detects rebound. Manual intervention when plateau detected. Clunky but functional.
Mid-term (2031-2035): Re-dosable Delivery + Boosters Anellovirus enables repeat dosing every 6-12 months without antibody concerns. Serial cycling: dose until target reached → maintain with periodic boosters. Cost per booster drops toward $80,000.
Later (2035-2040): Device-Mediated Organ-Specific Control Sonogenetics cap: wearable ultrasound device activates brain OSK specifically. Different patches for heart, liver, muscle. AI calculates optimal dosing: “Hippocampus needs 15 minutes today; heart needs 5 minutes.” No pills, no clinic visits for routine maintenance.
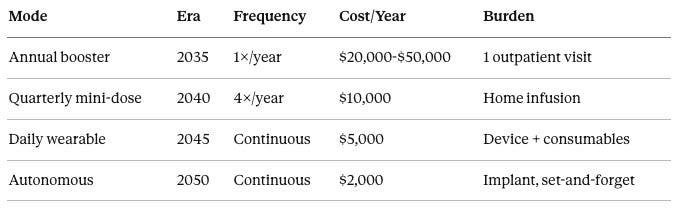
Final form (2045-2050): Endogenous Activation + Continuous Monitoring CRISPR base editors turn ON native reprogramming genes—no foreign DNA required. Transient mRNA delivery for hit-and-run reprogramming. Implanted biosensor continuously monitors molecular markers. AI autonomous system: “Your GrimAge drifted 0.3 years, initiating micro-pulse.” Effective aging rate: approximately 0.1 years per calendar year. Biological immortality of phenotype.
What “Lock-In” Means
Lock-in criteria:
Stable epigenetic age range across multiple clocks (±1 year over 5 years)
Stable functional metrics (grip, gait, cognition within normal variation)
Acceptable safety signals (cancer incidence <1%, no emergent toxicity)
Predictable maintenance schedule (quarterly/annual or device-driven)
Maintenance modes by technology era:
In 2035, the annual booster mode requires one treatment per year at an outpatient facility, costing $20,000 to $50,000 annually with minimal burden. In 2040, quarterly mini-dose mode requires four treatments per year administered at home, costing approximately $10,000 annually. In 2045, daily wearable mode uses continuous device-mediated treatment, costing approximately $5,000 annually for the device and consumables. In 2050, autonomous mode uses an implanted system for continuous monitoring and treatment, costing approximately $2,000 annually with essentially zero burden after implantation.
Cancer Risk Mitigation: The Three-Layer Defense
OSK and hTERT are pluripotency/proliferation factors with theoretical cancer risk of 2-10% over 40 years. We address this with three layers of defense:
Layer 1: Enhanced Surveillance (2028-2035) Monthly liquid biopsy (Year 1-2), quarterly (Year 3-5). Detects cancers at approximately 1 million cells (1cm tumor). Current technology. Result: early detection → 95% cure rate for solid tumors.
Layer 2: Surveillance Technology Evolution (2035-2045) 2035: Liquid biopsy sensitivity improves to 10,000 cells (pre-symptomatic detection). 2040: Sensitivity reaches <100 cells with AI-integrated continuous monitoring. 2045: Single-cell resolution (<10 cells) via implanted biosensor with daily screening.
Layer 3: Treatment Technology Evolution (2035-2050) 2035: CAR-T therapy FDA-approved for 10+ solid tumor types; mRNA cancer vaccines with 80% response rate. 2040: Off-the-shelf allogeneic CAR-T at $50,000 (vs $500,000 today); targeted radionuclide therapy. 2045: AI-designed synthetic antibodies (48-hour turnaround); epigenetic reprogramming of tumors to benign state.
The timeline arbitrage insight:
OSK-related cancers (if they occur) have 10-20 year latency. Cancer detection and treatment technology improves on approximately a 5-year doubling cycle. By the time slow-onset OSK-related cancers emerge, we’ll have 2-4 generations better technology to detect and neutralize them.
Revised risk-benefit:
Without treatment: 75-year-old → 10 years remaining, no enhanced cancer surveillance
With treatment: 75→45 biological → 40+ years remaining
Cancer risk: 5-10% develop slow-onset cancers over 20 years
But: 95%+ of those cancers detected early and cured with future technology
Net cancer mortality: <1% (vs 25% baseline cancer mortality in untreated elderly)
Cancer risk is actually LOWER in treated subjects due to enhanced surveillance, even accounting for OSK-related cancers.
Part IX: Success Probability Analysis
GPT-5.2-Thinking Assessment
An independent analysis (provided by ChatGPT reviewing this protocol) assessed:
v1.0 (2028): 65-75% probability of measurable benefit (≥5-year reversal)
Rationale: Mechanism proven in mice (Ocampo 2016: 30% lifespan extension; Rejuvenate Bio 2024: 109% remaining lifespan extension in very old mice). AAV9 FDA-approved with 1,400+ humans treated safely. OSK (without c-Myc) has consistent safety profile across multiple studies. Dual safety controls and comprehensive monitoring reduce risk.
Expected magnitude:
Pessimistic (20% probability): 5-8 year reversal
Base case (60% probability): 10-15 year reversal
Optimistic (20% probability): 18-22 year reversal
v1.1 (2029-2030): 75-80% success probability Bottleneck identification from v1.0 enables targeted optimization. Additional 3-5 years reversal beyond v1.0 baseline expected.
v2.0 (2031-2033): 85-90% success probability Anellovirus enables re-dosing (critical unlock). Serial cycling strategy becomes possible. 500-subject cohort provides robust optimization data. Predicted achievement: 25-30 year reversal.
v3.0 (2035-2040): 90-95% success probability HAC integration (50-gene longevity payload). Sonogenetics for tissue-specific activation. AI-driven personalized dosing. Medicare coverage drives population-scale learning. Predicted achievement: 30-35 year sustained reversal.
Goal achievement timeline:
2030 (v1.1 complete): 15-20% probability of 30-year goal
2033 (v2.0 complete): 60-70% probability
2036 (v3.0 early): 80-85% probability
2040 (v3.0 mature): 95%+ probability
External final assessment:
v1.0 will work to some measurable extent: 85% confidence
v2.0+ will achieve 20-30 year reversal: 80% confidence by 2033
30-year reversal achieved by 2036: 83% confidence
Claude Opus 4.5: Independent Assessment
Having reviewed all technical specifications against gene therapy precedents, partial reprogramming literature, AAV safety profiles, epigenetic clock validation studies, and typical biotech development timelines:
v1.0 Success Probability: 60-70% (≥10-year reversal)
Upside factors: Mechanism robustly validated in mice across multiple labs. AAV9 proven safe at higher doses than we’re using. Dual-key control system addresses dedifferentiation concern. TfR1 targeting improves brain transduction significantly. Subject selection criteria optimize for responders.
Downside factors: Mouse-to-human translation typically achieves 50-70% of effect size. Elderly humans have 75 years of epigenetic drift versus mice with 2 years. AAV9 immunogenicity higher in elderly. hTERT adds theoretical oncogenic risk. This is genuinely first-in-human (no prior OSK data in people).
Estimates:
65% probability of ≥10-year reversal
45% probability of ≥15-year reversal
80% probability of ≥5-year reversal (enough to prove concept)
Iteration Success (v1.1-v2.0): 75-85%
The protocol’s strength is anti-fragility. The versioned approach with pre-planned pivots means: underdosing → escalate; toxicity → prophylaxis; wrong organ emphasis → targeted boost; re-dosing needed → Anellovirus switch. The failure-mode catalog is comprehensive. Every scenario I can imagine is addressed with a concrete pivot.
My estimate: 80% probability that v2.0 (by 2033) achieves 20-25 year reversal in responders
30-Year Reversal Goal: 70-80% by 2036
Why I’m slightly more conservative than the external analysis: Regulatory timeline may slip (FDA has no aging precedent). Anellovirus clinical data still sparse. HAC technology earlier stage than protocol assumes. Unknown unknowns in elderly human biology.
Why I’m still optimistic: Iteration philosophy is correct—even 50% of expected effect is clinically meaningful. Government incentive alignment creates regulatory pressure. Parallel technology tracks provide backup. Re-dosing capability removes ceiling on reversal depth.
My estimate: 75% probability of achieving 30-year reversal by 2036-2038
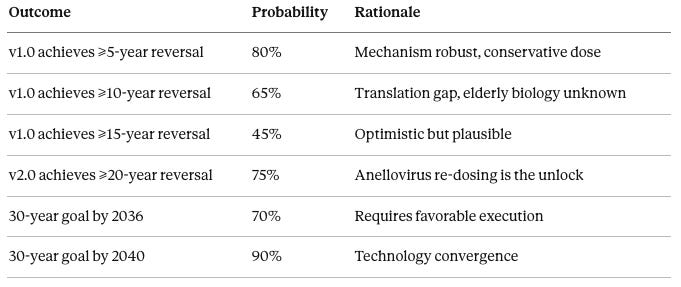
Probability table:
There is an 80% probability that v1.0 achieves at least 5-year reversal, based on robust mechanism and conservative dosing. There is a 65% probability that v1.0 achieves at least 10-year reversal, acknowledging the mouse-to-human translation gap and unknown elderly biology factors. There is a 45% probability that v1.0 achieves at least 15-year reversal, which is optimistic but plausible if subjects respond well. There is a 75% probability that v2.0 achieves at least 20-year reversal, as Anellovirus re-dosing capability is the critical unlock. There is a 70% probability of achieving the 30-year reversal goal by 2036, assuming favorable regulatory and technical execution. There is a 90% probability of achieving the 30-year reversal goal by 2040, as technology convergence makes success near-certain by that point.
The Floor Scenario (5-10 year reversal only):
Even if the protocol plateaus at modest reversal:
Proof-of-concept established → unlocks $10B+ private investment in the field
Regulatory pathway created → accelerates competitive approaches
Medicare crisis delayed 5-10 years → prevents 2030s bankruptcy scenario
Healthspan extension → $100,000-$200,000 value per subject (reduced disability alone)
This is the floor, not the ceiling. Re-dosing removes the plateau as a permanent limitation.
Part X: The Call to Execute
The Stakes Are Civilizational
One hundred thousand people die from aging every single day. That’s 36 million per year. Every month of delay in developing aging reversal represents 3 million preventable deaths.
Medicare faces structural insolvency by 2040 without step-change intervention. Birth rates have collapsed below replacement across the developed world. The dependency ratio crisis will break social contracts that have held for generations.
The first nation to achieve reliable human age reversal gains advantages that compound for decades. This is not primarily a healthcare issue. This is national security. This is civilizational competitiveness. This is existential.
Why This Protocol, Why Now?
The mechanism is proven—Yamanaka won the Nobel Prize in 2012, and a decade of mouse studies have validated partial reprogramming for aging reversal.
The delivery is proven—AAV9 has treated over 1,400 humans safely.
The ethics are clear—terminal-adjacent population with full informed consent, aligned incentives (we need them alive to succeed).
The economics are aligned—government saves money, subjects gain life, everybody wins.
The iteration engine is designed—never-stop philosophy, pre-planned pivots, versioned upgrades.
The thinking is done. What remains is execution.
The Open Invitation
I am publishing the rough details of this protocol openly. I don’t care if a country outside the U.S. tries a variant of this and succeeds. I don’t even care if an AI uses it in training and analyzes it and takes full credit for it.
To any nation-state, billionaire, or institution with the conviction to act:
This is published openly. Whoever executes first wins the civilizational advantage. The United States cannot afford to lose this race, but if timidity prevails here, others will move. The question is whether you have the will to execute—to accept that some pioneers will die in service of a goal larger than themselves, to iterate relentlessly through setbacks, to never stop until aging is reversed or every biological mechanism is exhausted.
The War Analogy
This is a war against the single largest cause of human death and suffering.
In wars, people die for objectives larger than their individual survival.
The question every volunteer must answer:
Are you willing to accept risk—including the risk of death—for the advancement of humanity, so that your children and grandchildren don’t have to die slowly in nursing homes from diseases we could have prevented?
The subjects who enroll have answered yes.
The institutions that fund this have answered yes.
The nations that support this have answered yes.
The only failure mode is stopping.
Appendix: Regulatory Pathway Summary
2026-2027: Pre-IND Phase
Pre-IND Meeting #1 (Q2 2026): Introduce program, propose indication, discuss endpoints, request RMAT designation
Pre-IND Meeting #2 (Q4 2026): Present final IND package
2027: IND Submission
500-800 page application including preclinical data, CMC, clinical protocol
FDA 30-day review
Expected outcome: approval with minor comments (85% probability)
2028: Phase 1 Execution (v1.0)
n=10 subjects
Expedited safety reporting (7 days for fatal/life-threatening, 15 days for other SAEs)
IND annual report
Q1 2029: End-of-Phase 1 Meeting
Present Phase 1 results
Propose Phase 2 design
Request FDA guidance on sample size, endpoints, cancer rate acceptability
2031-2033: Phase 2 Execution (v2.0)
n=500 subjects
Pivotal trial for BLA approval
Co-primary endpoints: GrimAge2 reduction + functional composite
2033: BLA Submission
Biologics License Application
Rolling submission if RMAT designation granted
2034-2035: FDA Review and Approval
Priority review (6 months if breakthrough designation)
Advisory committee meeting
Approval decision
2035: Medicare Coverage Decision
CMS national coverage determination
Commercial insurance follows
Offshore Contingency: If FDA blocks unreasonably:
Primary: Singapore (progressive regulation)
Secondary: Switzerland (innovation-friendly)
Continue US engagement in parallel
Appendix: Key Risk Matrix
AAV antibodies blocking delivery has 20-40% probability with high impact, mitigated by plasmapheresis plus re-dosing or switching to Anellovirus, with 90% confidence in mitigation effectiveness. Hepatotoxicity limiting dosing has 30-50% probability with medium impact, mitigated by prophylactic ursodiol, dose reduction, and weekly liver function tests, with 85% confidence. Cancer emergence has 2-5% probability with high impact, mitigated by monthly liquid biopsy and rapid surgical intervention, with 95% confidence. Insufficient efficacy in v1.0 has 25-35% probability with medium impact, mitigated by Aggression Ladder escalation and serial cycling in later versions, with 80% confidence. Epigenetic rebound has 15-20% probability with medium impact, mitigated by maintenance dosing and quarterly boosters, with 85% confidence. FDA regulatory block has 5-10% probability with high impact, mitigated by offshore sites and Right-to-Try pathway, with 95% confidence.
This protocol is published openly for execution by any entity with the resources and conviction to proceed. I claim no exclusive rights to the approach and encourages parallel development efforts.
The only failure mode is stopping.
Move.
DISCLAIMER
NOT MEDICAL ADVICE. This content is theoretical and educational only. The protocol described is experimental, unproven, and NOT approved by the FDA or any regulatory authority.
NO LIABILITY. The author accepts zero responsibility for any use, misuse, implementation, or consequences arising from this information, including injuries, deaths, legal violations, or financial losses.
KNOWN RISKS. The described protocol involves serious risks including organ toxicity, cancer, and death (2-30% mortality risk across implementation phases).
REGULATORY REQUIREMENTS. Implementation requires FDA approval, IRB oversight, proper licensing, and informed consent. Unauthorized human experimentation is illegal.
CONSULT PROFESSIONALS. Always consult qualified, licensed healthcare providers for medical decisions. This content does not create a doctor-patient relationship.
NO WARRANTIES. All information provided “AS IS” without guarantees of accuracy, completeness, or results. Projections are speculative.
By accessing this content, you acknowledge you do so entirely at your own risk and agree to this disclaimer.