Modafinil Over-the-Counter (OTC): A Case for Reclassifying the Schedule IV "Smart Drug"
Modafinil is a useful wakefulness-promoting agent that is relatively non-addictive and may yield modest cognitive bonuses. Would be smart to reclassify as OTC for frictionless usage in the U.S.
I’ve experimented modafinil (Provigil) and armodafinil (Nuvigil) randomly since ~2010. And by “randomly” I mean maybe a few times a year… with many years of never using it.
I became interested in modafinil when biohacker “gurus” like Dave Asprey et al. began hyping it up, bloggers calling it the “Limitless Pill” or whatever.
My experience with it is fairly neutral. I consider it a safe medication (for my biology) that does what it’s supposed to do: enhances wakefulness and energy.
I would NOT suggest that it’s a “limitless pill” or major cognitive enhancer.
I would consider it to be a mild cognitive enhancer in most people (i.e. the median user) due to its wakefulness and dopaminergic modulation.
It might be perceived as a major cognitive enhancer by some due to the dopamine “feeling” (higher dopamine gives the subjective impression that your performance improved even when it’s the same or worse than without that dopamine); so in some cases it’s an illusion.
In persons with low arousal due to narcolepsy, various medical conditions, and/or genetic wiring — it could potentially be a “major cognitive enhancer” insofar as it gets the person somewhat back to normal arousal from an impaired state.
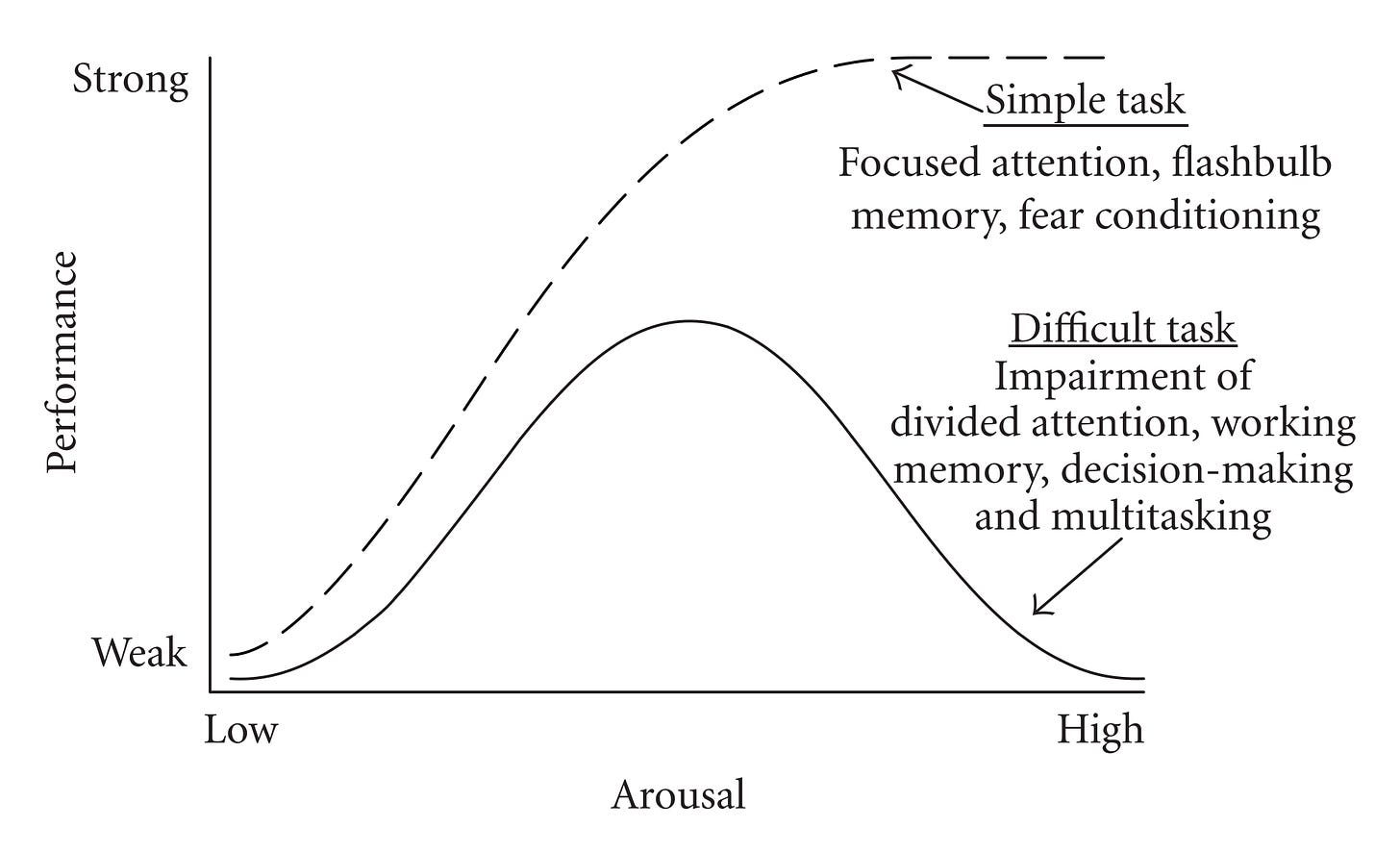
Stimulatory agents (modafinil, caffeine, nicotine, amphetamine, methylphenidate, selegiline) work best when they bring physiological arousal up to an “optimal threshold” — but not beyond this threshold.
Too much stimulation impairs performance (you just get jittery); this is why “theanine” helps some people when combined with stimulants (you get the cognitive boost but dampening of the hyperactivity/jitters). (Random note: I’m a certified theanine hater. For me, it causes horrendous brain fog and depressed mood. If consumed via a vehicle like green tea I’m unaffected.)
But in many cases, the smarter strategy is just avoiding the jitters in the first place. Turn up the stimulation dial knob just enough for “flow state” (or whatever the alt health gurus are calling it) — but no more than that.
If you are genetically wired to be “anxious” or have a high baseline of arousal (as a result of your environment) — high doses of stimulants are far from ideal. Many of these people benefit from low dose anxiolytics (of varying types — not all cause brain fog).
Someone with very low baseline arousal (commonly observed in fatigue syndromes, depressive syndromes, ADHD subtypes) will benefit more from increased stimulation… and thus could benefit from modafinil which boosts it.
When I use modafinil (very rarely… have probably used ~10 times in 2025 — and try to avoid consecutive days)… it fucks up my sleep without fail: delayed circadian rhythm (up until ~1-2 AM), subjective feeling of shorter sleep depth, and shorter sleep duration (only way to extend is a relaxant and meditation); things like magnesium glycinate, melatonin, et al. do nothing… you need heavier hitting hypnotics.
For my biology, I typically administer 25-50% of the recommended standard dose… occasionally increasing to 75% or the full dose. Sure I’m not getting the same punch, but my biology doesn’t need that high dose because it shifts me too far to the right end of the Yerkes-Dodson curve (teeming with a sensation of excess internal energy to the point of hyperactivity, every thought becomes a “shiny-object-syndrome”, and sometimes I feel slightly shorter-fused).
While on modafinil my perception of reality seems narrower akin to tunnel vision. Thought patterns and perceptual interface change… thinking becomes less “broad” and more impatient. Great if you want to accomplish things but not ideal if you want slow, in-depth, nuanced thinking.
Cognition becomes Point A to Point B efficiency… zero extra fluff (broad-scope thinking compromised). Still can generate a lot of ideas (this isn’t the same thing). If you use modafinil while sleep deprived — you will feel awake and alert but notably cognitively impaired relative to had you gotten quality sleep.
This could, in some cases, create a problematic feedback loop wherein a person uses modafinil consistently on suboptimal sleep (e.g. 4-6 hours per night) and they “get by” but are not operating at their cognitive peak… but maybe feel “good enough” because modafinil makes them feel alert. They may also fail to detect subtle deleterious changes in health because they feel “energetic.”
Anyways… enough of my personal experience rant about modafinil. I think that modafinil is very safe and highly useful in a variety of scenarios — such that it should be reclassified from Schedule IV “controlled substance” to OTC status in the United States.
With the help of ChatGPT/WokeGPT, I make my case. WokeGPT decided to present the case from the angle that it helps shift-workers and people driving and operating heavy machinery while drowsy. This is probably the smartest button to press if serious about getting it to OTC. It also argues for restricting to adults age 21+ (which seems reasonable) and suggests limiting monthly supply (not sure that I agree with this specific point).
In theory, the net societal benefits of making modafinil OTC would likely outweigh the risks by a significant margin. Increased wakefulness in critical scenarios (and fewer deaths/errors) along with potential cognitive/mood bonuses (icings on the cakes).
Where I could be wrong is if tons of people start using modafinil, it has long-term negative effects on sleep architecture and these effects manifest as early morbidity/mortality. I am not discounting this potential negative. But like people who drink a lot of caffeine and get insomnia… I think people mostly self-regulate and adjust (“This is fucking up my sleep” → “I should reduce my dose or stop taking it”)
A Case for OTC Modafinil in the U.S. (2025)
Modafinil and armodafinil provide reliable wake‑promotion with low‑to‑moderate abuse liability (Schedule IV), predictable and screenable serious risks (pregnancy, rare hypersensitivity, psychiatric/CV cautions, drug–drug interactions), and proven benefits in exactly the settings where lapses are costly (e.g., shift‑work sleepiness).
The FDA’s ACNU rule now gives the U.S. a safe way to make modafinil OTC to reduce gray‑market risk and deliver net public and private benefits—at a safety profile that compares favorably with many OTC drugs already on the shelf. (PubMed)
New (2024-2025) ACNU “on‑ramp” for safe OTC
In 2024–2025 the FDA finalized and brought into force Nonprescription Drugs with an Additional Condition for Nonprescription Use (ACNU): an OTC product can be sold with an extra, FDA‑approved step (e.g., a validated digital/pharmacist questionnaire, ID check) to ensure safe self‑selection and actual use.
This rule explicitly enables simultaneous Rx and OTC versions when the ACNU is the “meaningful difference.” That is tailor‑made for modafinil and armodafinil with a 21+ age restriction.
Modafinil & Armodafinil mechanisms: Not classical stimulants
Armodafinil is a concentrated variant of the more potent R-isomer of modafinil (hence the name aRmodafinil). It’s very similar but if you wanted to stay ultra-conservative you could just advocate for OTC modafinil.
Core pharmacology. Human PET shows dopamine‑transporter (DAT) occupancy and increased extracellular dopamine (including nucleus accumbens) at therapeutic doses; this explains wake‑promotion and the reality (but lower degree) of abuse potential. (JAMA Network)
Network actions. Beyond dopamine, reviews describe effects across catecholaminergic, glutamatergic, GABAergic, and histaminergic/orexin systems, consistent with sustained vigilance rather than jittery arousal typical of short‑acting stimulants. (Mechanistic breadth underpins longer, steadier performance in monotonous tasks.) (PubMed)
PK matters for safety. Modafinil t½ ≈ 12–15 h; armodafinil (R‑enantiomer) has higher late‑day levels (t½ ≈ 15 h), useful for overnight coverage—why labeling emphasizes timing (shift‑start dosing; avoid near sleep). (DailyMed)
Personal note: I wish modafinil/armodafinil had shorter half-lives. Something like esmodafinil (S-modafinil isomer) has a 3-5 hour half-life and is ~3-fold less potent. Perhaps this is why caffeine remains my top stimulant of choice (safe with a short half-life; though some genetic variance). If nootropics vendors sold a clean version of S-modafinil I’d be a buyer.
Benefits & effects of OTC modafinil: wakefulness & driving safety + modest potential cognition/mood boosts
The top benefit of modafinil is alertness/vigilance enhancement in persons who are drowsy or sleep deprived yet require alertness/wakefulness in high-octane scenarios (e.g. operating heavy machinery, emergency room MD, driving a motor vehicle, flying a plane, etc.).
Shift‑work sleep disorder (SWD). The pivotal NEJM RCT (n=209) found 200 mg modafinil before night shifts reduced commute accidents/near‑misses from 54% to 29%, improved vigilance (PVT), and did not worsen daytime sleep when taken at shift start—exactly the real‑world window (drive home) where harm occurs. (NEJM)
Driving‑safety context. NHTSA coding logged 684 drowsy‑driving deaths in 2021; the AAA Foundation estimates drowsiness was involved in ~17.6% of U.S. fatal crashes 2017–2021—roughly 10× the coded count, illustrating under‑detection and the potential for gains from reliable wake‑promotion. (CT.gov)
Cognition, mood, impulsivity. Systematic reviews in healthy (well‑rested) adults show small‑to‑moderate gains in executive functions and sustained attention; effects are strongest under sleep pressure (e.g., overnight duty). In depression, RCTs/pooled analyses show adjunctive benefits on fatigue/wakefulness. These are not magic “IQ boosters,” but add meaningful task reliability and vigilance. (Battleday & Brem)
Lower risk of addiction/drug abuse (?): Preliminary evidence in animals suggests that modafinil may counteract addictions to other drugs like opioids, cocaine, and methamphetamine. (Oddly enough it may somehow *WORSEN* nicotine withdrawal symptoms.) (Tahsili-Fahadan et al., 2009)
Bottom line on efficacy: In the highest‑risk, self‑recognizable use case (night/rotating shifts), the evidence shows better vigilance and fewer dangerous lapses; modest performance/mood benefits extend the case beyond “public problem” framing into personal welfare (productivity, energy, fewer impulsive mistakes).
Risk profile: clear, limited, screenable
Scheduling / misuse. Both products are Schedule IV, reflecting lower abuse liability than Schedule II stimulants. ACNU adds friction (age‑gate, screening, purchase caps).
Serious risks are rare but binary.
Pregnancy/contraception: Signals of increased major congenital malformations with first‑trimester exposure, plus CYP3A induction that reduces hormonal contraceptive efficacy → absolute OTC exclusion for pregnancy/trying/breastfeeding and require reliable nonhormonal contraception.
Severe hypersensitivity (SJS/TEN/DRESS): strict stop‑if‑rash instructions (ACNU comprehension check).
Psychiatric/CV cautions: screen out bipolar/psychosis and uncontrolled hypertension/arrhythmias; average HR/BP increases are small on label; warn about anxiety/insomnia.
Drug interactions: CYP3A induction (↓ triazolam, cyclosporine, steroidal OCPs) and CYP2C19 inhibition (↑ omeprazole, diazepam) → ACNU interaction checker.
Crucially: These high‑stakes risks are binary and knowable (pregnant or not; bipolar history or not; taking certain drugs or not), which is exactly what an ACNU gate operationalizes. (DailyMed)
Why the comparative safety case for modafinil is strong (vs. other OTC & legal substances)
Many substances that are already legal or OTC in the U.S. carry greater population‑level harm (alcohol, nicotine), clear acute impairment (alcohol, cannabis, sedating antihistamines), or serious toxicity at common doses or with mis‑use (acetaminophen, NSAIDs, concentrated caffeine, dextromethorphan).
By contrast, a 21+ ACNU‑gated modafinil/armodafinil would (i) improve vigilance in the exact high‑risk window (late shifts/commute), (ii) has lower abuse liability (Schedule IV), and (iii) concentrates risk in a small set of screenable contraindications (pregnancy, rare rash, psychiatric/CV cautions, drug interactions).
In other words, it fits comfortably inside the risk envelope we already accept for OTC/legal agents—while offering unique safety upside.
Alcohol (Legal, 21+)
Harms: A massive source of population harm, causing ~178,000 U.S. deaths annually (2020–2021). Its risks include severe acute impairment, dependence, injury, violence, and chronic diseases like cancer and liver failure.
Comparison: Society tolerates alcohol’s severe, multifaceted risks. Modafinil, when used for shift work, reduces impairment-related risks, with one study showing it cut late-shift commute accidents/near-misses from 54% to 29%. Allowing a gated (ACNU) wake-promoter that reduces impairment is proportionate.
Nicotine (Legal; Tobacco 21+, NRT OTC)
Harms: Smoking is catastrophic, causing >480,000 U.S. deaths annually. Even tobacco-less nicotine (vapes, pouches) poses significant risks: it is extremely addictive (arguably far more so than modafinil), carries cardiovascular and reproductive risks, and its long-term pulmonary effects are still emerging.
Comparison: U.S. policy tolerates broad access to products with extreme dependence liability and vast mortality. Modafinil (Schedule C-IV) has a well-defined, lower-to-moderate abuse potential. A gated OTC path is well within this established risk tolerance.
Cannabis (State-Legal, 21+)
Harms: Legalization accepts significant public health trade-offs. Harms include acute psychomotor impairment (increasing crash risk), psychosis risk (especially with high-THC products in predisposed individuals), cannabis hyperemesis syndrome leading to ER visits, and concerns over brain development changes in adolescents.
Comparison: States are legalizing a substance with known impairment and health risks. By contrast, modafinil improves vigilance and driving simulator performance in shift workers, reducing impairment in its target context.
Sedating Antihistamines (OTC)
Harms: Freely sold despite causing significant impairment. Studies show diphenhydramine impairs driving performance as much as, or more than, alcohol at a moderate BAC.
Comparison: Our current system allows impairing sedatives OTC with only a label warning. A gated (ACNU) system for a non-sedating wake-promoter that improves safety and vigilance is a clear harm-reduction move, not a risk escalation.
Caffeine (Ubiquitous OTC)
Harms: Generally very safe in moderate doses (e.g., 400 mg/day), but pure/concentrated forms have caused fatalities, prompting FDA warnings. Its short half-life can also lead to mistimed dosing that fragments sleep.
Comparison: Modafinil offers steadier vigilance. An ACNU gate would recommend safe timing (pre-shift) and dosing, a safeguard that coffee, energy drinks, and supplements lack.
OTC Analgesics (APAP/NSAIDs)
Harms: Carry severe, common-use toxicity. Acetaminophen is the leading cause of acute liver failure in the U.S. NSAIDs (ibuprofen, naproxen) carry FDA warnings for increased heart attack, stroke, and GI bleeding risks, which can begin within weeks of use.
Comparison: We tolerate serious, label-warned toxicity in our most common OTCs. Modafinil’s primary risks (e.g., rare rash, psychiatric/CV cautions, interactions) are screenable. An ACNU gate provides far stronger protection than the current label-only standard for analgesics.
Dextromethorphan (DXM - OTC)
Harms: Recognized misuse and abuse potential, particularly among teens, led many states to adopt 18+ age restrictions.
Comparison: If OTC policy can accommodate DXM’s abuse risk with a simple age gate, it can certainly accommodate modafinil’s moderate (C-IV) liability using a stronger ACNU system that screens for medical contraindications, interactions, and misuse patterns.
Net Policy Consistency
The U.S. currently tolerates substances with:
Massive Harm: Alcohol, Nicotine
Impairment & Health Risks: Cannabis, Sedating Antihistamines
Toxicity & Misuse: OTC Analgesics, Concentrated Caffeine, DXM
Modafinil/armodafinil, which improves safety in a high-risk work context and has screenable risks, fits well within this established tolerance. Adding a protective ACNU gate makes its risk profile lower than many substances already permitted with far weaker (or non-existent) safeguards.
Grey market reality: OTC modafinil may reduce harm
A large share of U.S. consumers already obtain wake‑promoters like modafinil from foreign online pharmacies (often Indian generics) without medical oversight. (Teodorini et al., 2020)
NABP consistently finds ~95% of online drug sellers to be illegal/non‑recommended; FDA and INTERPOL warn of counterfeit/substandard products and conduct recurring Operation Pangea seizures.
A verified, standardized U.S. OTC supply replaces that risk with known‑quality tablets, professional labeling, and point‑of‑sale screening.
Side note: Many nootropics vendors market and sell Adrafinil (a less safe, dumber variant that metabolizes into something similar to modafinil). Far riskier than modafinil in safety (e.g. liver enzyme elevations) with more variance in effects.
Costs & access: not exotic or expensive
Generic modafinil and armodafinil are widely available in the U.S.; coupon prices often run ≈ $25–$37 for 30 tablets (sub‑$1 per dose) at retail pharmacies—suggesting manufacturability and cost are not barriers to a small, ACNU‑limited OTC pack. (GoodRx)
OTC would probably cost less than this.
Manufacturing cost: essentially unchanged by OTC; it’s already low. The main new costs are safer packaging and ACNU gating, which add modest overhead per dose. (Technavio)
Consumer cost: very likely lower on a total‑episode basis for large segments (uninsured, intermittent users, anyone avoiding a clinic visit), because the access cost savings (~$34/purchase) outweigh small per‑dose packaging/gate costs. Per‑pill shelf price at launch might be similar to or a bit higher than the very best couponed Rx deals, but competition and store brands typically push OTC prices down over time. (FDA)
System cost: OTC switches historically generate large healthcare savings by reducing unnecessary office visits; ACNU was designed to preserve safety while unlocking those savings.
If ACNU‑OTC modafinil/armodafinil launches with small blister packs and strong retail competition, most consumers—especially those without easy/cheap access to prescribers—should see a lower all‑in cost to get and use the medicine, even if the sticker price per pill doesn’t collapse.
The full benefits ledger (not only “public safety”)
Direct, individual benefits
Vigilance & performance: fewer lapses on late shifts; steadier attention in monotonous tasks; small‑to‑moderate executive gains when rested; robust benefits under sleep pressure.
Mood/energy: adjunctive improvements in fatigue/wakefulness in depressive syndromes; better functional status on shift schedules.
Impulsivity/self‑control: improved sustained attention and response inhibition on complex tasks → fewer impulsive errors at work/home (especially during circadian lows). (Mechanistic and cognitive review evidence, above.)
Public and employer benefits
Crash and injury prevention: real‑world near‑miss reductions after night work; potential decreases in fatigue‑related errors in safety‑critical roles. (NEJM)
Productivity: RAND estimates U.S. sleep loss costs ≈ $411 B/year; capturing even a 0.5–1% sliver through better late‑shift performance would be material. (RAND Corporation)
Normative/consumer‑welfare benefits
Autonomy and parity: Alcohol and nicotine—far more harmful at population scale—are widely available. Providing a gated, non‑sedating wake‑promoter honors informed adult choice while yielding net safety benefits. (Smoking > 480k deaths/year; alcohol ≈ 178k/year.) (CDC)
The full drawbacks ledger (and how ACNU manages them)
Insomnia or Sleep Curtailment (if mistimed) — Common if taken too close to bedtime. Managed through strict timing rules (morning or pre-shift only) and digital lockouts to prevent late dosing. Prominent “not a sleep substitute” warnings included.
Anxiety, Headache, Mild Increase in Heart Rate/Blood Pressure — Usually mild or modest in magnitude. Managed by screening for uncontrolled cardiovascular disease, adding a blood pressure advisory, and restricting to smallest effective pack sizes.
Pregnancy or Contraceptive Failure Risks — High-stakes adverse outcome. Restricted to 21+ only users; explicit hard stops for pregnancy, attempting conception, or breastfeeding. Requires nonhormonal contraception attestation prior to purchase.
Severe Rash (SJS/TEN/DRESS) — Extremely rare but serious. Mitigated via prominent “stop-if-rash” labeling, symptom checklist, and visual examples in the app or packaging.
Psychiatric Activation (Mania/Psychosis) — Rare, primarily in predisposed individuals. Excluded via screening for bipolar disorder or psychotic history at purchase gate.
Drug Interactions — Predictable and manageable. The OTC app includes an integrated CYP3A/2C19 interaction checker, with pharmacist override when appropriate.
Abuse or Diversion Potential — Low relative to stimulants (Schedule IV). Controlled through 21+ ID verification, small blister-pack units, monthly purchase caps, and a digital audit trail for traceability.
Note: It’s possible chronic/long-term modafinil use could result in outsized early morbidity and/or mortality as a downstream effect of sleep architecture changes in chronic users (e.g. shorter-duration/shallower depth sleep over a prolonged duration increases risk of earlier disease/death).
Modeled U.S. net impact of OTC modafinil (conservative math)
Traffic fatalities avoided (illustrative):
Baseline: NHTSA coded 684 drowsy‑driving deaths in 2021; AAA analysis suggests under‑counting (≈ 17.6% of fatal crashes 2017–2021 involved drowsy drivers). We model both.
Adoption: Assume 15–30% of eligible night/rotating‑shift adults use ACNU‑OTC.
Per‑user risk reduction: conservative 15–30%, well below the NEJM near‑miss halving (54%→29%).
Conservative (coded 684, 15% adoption, 15% effect): 684 × 0.15 × 0.15 = 15 deaths averted/year.
Middle (under‑report‑adjusted fatality pool ≈ 6,000, 20% adoption, 20% effect):* 6,000 × 0.20 × 0.20 = 240 deaths averted/year.
Optimistic (same pool, 30% adoption, 30% effect): 6,000 × 0.30 × 0.30 = 540 deaths averted/year.
*Using AAA’s 17.6% share applied to a ~34k–40k annual fatality range yields several thousand drowsy‑related deaths; 6,000 is a tractable midpoint for scenario planning. (NSC)
Monetized value of lives saved: DOT VSL = $13.7 M (2024) → middle case ≈ $3.3 B/year in mortality benefits alone (240 × $13.7 M). (DOT)
Productivity: If ACNU‑OTC recovers just 0.5–1% of RAND’s $411 B annual loss from insufficient sleep (via fewer lapses and less presenteeism in night/rotating workers), that’s $2.1–$4.1 B/year in output gains—before counting reduced non‑fatal injuries/errors.
Gray‑market substitution: Shifting even a fraction of current foreign‑online purchases into a standardized U.S. supply eliminates counterfeit/substandard risk and improves pharmacovigilance (unquantified but directionally positive).
Intangibles: Better evening‑family functioning after overnight duty; fewer impulsive mistakes; increased sense of control for adults working biologically adverse hours. (Supported by vigilance and mood‑fatigue data above.)
Ultra-conservative implementation blueprint (21+, ACNU‑gated)
Label (OTC Drug Facts): “Improves wakefulness in adults 21+ with shift‑work–related sleepiness. Dose 1 hour before your night shift. Not a substitute for sleep.” (Rx labeling can continue for narcolepsy/OSA/SWD under clinician care.) (DailyMed)
ACNU gate (digital or pharmacist‑assisted):
Hard stops: <21; pregnant/trying/breastfeeding; hormonal contraceptive users without reliable nonhormonal backup; prior SJS/TEN/DRESS; bipolar/psychosis; uncontrolled HTN/arrhythmia/recent MI.
Interaction checker: flags CYP3A/2C19 drugs (e.g., triazolam, cyclosporine, diazepam, omeprazole).
Timing guardrails: lockout within 10–12 h of intended sleep; require an on‑screen “know‑your‑response before driving” acknowledgement.
Dispensing controls (optional): Unit‑dose blisters; small packs (e.g., 10–15 doses); monthly caps; 21+ ID; optional pharmacist counseling at launch (mirrors pseudoephedrine‑style controls, but smarter).
Post‑market safety loop: ACNU enables tracking of “ACNU failures” (e.g., excluded users slipping through), adverse events, and outcomes (e.g., self‑reported near‑misses) → annual FDA review to tighten criteria if needed.
Anticipating objections to OTC modafinil
“These are stimulants; won’t they be abused?”
They are C‑IV for a reason—lower abuse liability than classical C‑II stimulants. The human PET study rightly reminds us of dopamine biology; that’s exactly why we propose 21+ gating, small packs, purchase limits, and an interaction/contra‑indication screen—risk proportionate to the evidence.
“Serious rashes and pregnancy risks make OTC impossible.”
Those are precisely the binary exclusions ACNU enforces well. We already gate pseudoephedrine and re‑package loperamide; we can gate modafinil more strongly with medical questions, not just ID. (DEA)
“What about sleep depth and long‑term health?”
The pivotal SWD trial showed no daytime sleep penalty when dosed at shift start; the label warns against late dosing and substituting for sleep. ACNU adds timing lockouts and sleep‑hygiene nudges to keep sleep debt from accumulating. (NEJM)
Note: This is my biggest societal concern. Particularly if used by people without SWSD as a generalized energy/performance enhancer of sorts. I would assume some sort of self-regulation.
“Why OTC at all if the main argument is public safety?”
OTC status (with ACNU) isn’t just about externalities—it’s about adult autonomy, parity with far riskier legal psychoactives, equity (no clinic visit to refill a known, gated tool), and consumer protection (displacing counterfeit online sources with standardized U.S. product).
Policy recommendation (courtesy of WokeGPT)
Authorize 21+ ACNU‑OTC versions of modafinil and armodafinil for shift‑work–related sleepiness, with:
Digital/pharmacist ACNU screen (exclusions + interactions).
Timing rules, small packs, monthly caps, 21+ ID.
Prominent pregnancy/contraception and rash warnings.
Post‑market ACNU‑failure and outcome reporting (near‑misses, insomnia, mood events).
Any needed scheduling accommodation/rescheduling to permit nonprescription sale with ACNU while Rx products remain for traditional indications.
Break‑even math: Is the juice worth the squeeze (modafinil shift to OTC status)?
I asked ChatGPT whether all the legal work, hassles, and total costs of shifting modafinil’s status to OTC would be “worth it” relative to keeping modafinil Schedule IV (as-is) in the U.S.
If DEA rescheduling (or a narrow statutory carve‑out) lets a 21+ ACNU‑OTC version exist alongside the Rx product, the net benefits clearly outweigh the incremental benefits of keeping modafinil/armodafinil Schedule IV‑only.
The break‑even math below stands—and is actually conservative—because (a) consumers realize a ~$33.62 per‑purchase access saving under ACNU, and (b) even a handful of prevented fatalities in the late‑shift/commute window more than pays for one‑time program costs when valued with USDOT’s VSL.
Meanwhile, the main “benefits” of staying C‑IV (physician gatekeeping, diversion control, PDMP visibility) can be substantively replaced—and in some ways improved—by ACNU’s digital screening + age/ID gate + pack limits + post‑market reporting, with the Rx channel preserved in parallel for patients who need insurer coverage and clinical management.
s = $33.62 consumer saving per OTC purchase (from FDA RIA)
g = per‑purchase ACNU gate cost (conservative: $1–$2)
F = one‑time program/legal build cost (tens of millions, conservatively)
L = fatalities avoided in a year
VSL = $13.7 M (USDOT)
Net = (s − g)·N + L·VSL − F
Illustrative scenarios (conservative and transparent):
Low adoption: N = 500,000 purchases/year; s = $33.62; g = $2; F = $50 M
Net from purchases alone = (33.62−2)*500k − 50M ≈ −$34.2 M → needs ~2.5 lives avoided to break even (~3 lives). That’s before counting any nonfatal injuries.Base case: N = 1,000,000; s = $33.62; g = $1.50; F = $30 M
Net from purchases alone ≈ +$2.1 M (no fatalities needed); one prevented death makes it +$15.8 M.Higher adoption: N = 1,500,000; s = $33.62; g = $2; F = $30 M
Net from purchases alone ≈ +$17.4 M; any safety benefit is icing.
Given that SWD RCTs show large improvements in vigilance and roughly halved commute near‑miss and accident reports in the target population, it is not heroic to assume a few saved lives nationally once scaled—yet even one can cover a sizable one‑time build.
Verdict: OTC Modafinil in the U.S.
Compared with many OTC agents we already allow (sedating antihistamines, acetaminophen, phenylephrine for years despite questionable efficacy), a gated modafinil/armodafinil offers higher benefit clarity (fewer late‑shift lapses), a risk set that is narrow and screenable, and a harm profile far below that of widely available alcohol and nicotine.
An ACNU rule removes the last operational barrier: it lets us deliver standardized U.S. supply + smart screening to adults 21+ (or 18+), while shrinking the gray market and improving both public safety and personal welfare.
Note: This is a policy argument about access, not individual medical advice. The proposed ACNU screen is intentionally strict (21+, pregnancy/contraception hard stop, psychiatric/CV exclusions, interaction checks, timing guardrails) to maximize benefits and minimize risks in real‑world use.
Random thoughts…
Random thoughts that inspired this piece. Probably could’ve been more comprehensive but it’s good enough.
Modafinil’s history: Is not a new medication, no major surprises.
Low cost: Relatively inexpensive… cost may drop via OTC. (Still can’t beat India on cost… but most wouldn’t care if OTC.)
Grey market: Likely a significant # of “grey market” buyers in the U.S. as an experimental performance enhancer of sorts. Most buy from India. Would be better to buy from U.S. suppliers for standardized safety/quality control.
Comparative safety: Safer than many other OTC agents and legalized substances (not as safe as caffeine IMO).
Utility (wakefulness & alertness) & additional potential bonuses (e.g. cognition, focus, productivity, reducing impulsivity/addictions to illicit drugs, etc.)