Mirror Life Moratorium: Are Mirror Bacteria Too Risky?
Objects in mirror are riskier than they appear... or are they?
Mirror life has been in the news because prominent researchers have called for a moratorium (a voluntary pause) on creating mirror bacteria, alongside broader public and policy deliberation. And we’re doing this in the shadow of: (1) COVID and the gain-of-function drama, plus (2) the He Jiankui CRISPR-babies backlash (with no publicly verified harm so far). So the default posture is more risk-averse than usual.
Also, most mainstream coverage is basically “risk, therefore pause.” It barely mentions the upside case (especially the non-obvious benefits), so I’ll put the upside on the table too.
And scientists were already operating in a risk-averse ecosystem to begin with: IRBs, bioethics committees, biosafety panels, funding gatekeepers—scientists are on a short leash with a shock collar.
This is part of the reason it feels like “nothing ever happens” in science… you read a science publication (e.g. ScienceDaily, EurekAlert, etc.) and think 10,000 disease cures are coming tomorrow… yet time passes… you wake up 10 years later and there’s mostly nothing except Ozempic. (Okay, that’s embellished, but the rate of progress is still turtle mode relative to the tech we already have—sequencing, automation, insane compute, AI, etc.).
I’ve made the case that most bioethicists are unethical and getting paid to guarantee more long-term pain/suffering for humanity than if we went full-steam ahead with experimentation. This is why people are going to Prospera for experimental gene therapies vs. testing them in the U.S.
Ideally the entire world should be an “Operational Warp Speed” for bioenhancement: aging reversal, IQ/cognition upgrades, trait customization, disease eradication (prioritized by magnitude-of-impact x efficiency).
That said, my armchair take on mirror life is that a moratorium makes logical sense at the moment in 2026 and seems like an overall smart move (catastrophic tail risk + competing alternative technologies vs. decent upside potential).
But I don’t want to blindly pile on and say “yeah a moratorium is really smart” without considering the logic of dissenting perspectives.
With mirror life the risk/benefit looks asymmetric—or at least that’s what the tea leaves read today. But not all scientists agree, and it’s also common for people to join the risk-averse “herd,” amplify it, and play it safe by default.
This piece is basically a guide to help you think about mirror life and the moratorium—and form your own opinion, not borrow mine or anyone else’s.
What Is Mirror Life?
Most essential biomolecules are chiral—they exist in 2 mirror-image forms that cannot be superimposed, like left and right hands.
All known life on Earth is homochiral: DNA and RNA are built from “right-handed” nucleotides, while proteins use “left-handed” (L-) amino acids. This uniformity is so complete that deviations are rare curiosities.
“Mirror life” refers to hypothetical organisms with inverted chirality—using L-nucleic acids and D-amino acids instead. Such organisms have never existed in nature and cannot arise through incremental evolution from existing life, because flipping chirality requires simultaneously inverting an entire system’s molecular machinery.
Mirror life isn’t “mirror bacteria” specifically. In principle it could mean mirror archaea, mirror fungi, mirror plants/animals—anything self‑replicating with inverted chirality.
People fixate on bacteria because it’s the most plausible first engineered mirror organism… and because once you can build one self‑replicating mirror microbe, you’ve basically opened the door to everything else.
Also: a “mirror virus that infects humans” doesn’t really work the way people intuitively imagine—viruses steal host machinery, so a mirror virus would need a mirror host (think “mirror phage infecting mirror bacteria,” not “mirror COVID”).
Crucially: No mirror bacteria or mirror organisms exist today.
The Congressional Research Service and the 38 scientists who published in Science in December 2024 emphasize that: (1) full mirror life remains technically infeasible and (2) is likely at least a decade away, requiring massive investment to overcome fundamental barriers.
What Actually Exists: Mirror Components vs. Mirror Organisms
The critical distinction—repeatedly emphasized in UK policy discussions and the Science perspective—is between:
Mirror components (D-proteins, L-DNA/L-RNA, mirror enzymes): These exist and have legitimate applications
Mirror organisms (self-replicating cells with fully inverted chirality): These do not exist
Key Technical Milestones
Milestones:
2016: First mirror polymerase (174 amino acids) demonstrated mirror DNA copying (Wang et al., Nature Chemistry)
2017: Thermostable mirror polymerase (352 amino acids) enabled mirror PCR (Xu et al., Cell Discovery)
2021: Largest synthetic protein ever made—90 kDa mirror Pfu polymerase assembled kilobase-length mirror gene and stored text in L-DNA stable for 1+ year in pond water (Fan et al., Nature Biotechnology)
2022: 100 kDa mirror T7 RNA polymerase transcribed all three ribosomal RNAs—”a key milestone toward building a mirror-image ribosome” (Xu & Zhu, Science)
2024: Automated flow synthesis produced 12 D-proteins in single runs—nearly one-third of all D-proteins ever reported (Callahan et al., Nature Communications)
What’s Still Missing for Mirror Organisms
A functioning mirror bacterium would require:
A complete mirror translation system (mirror ribosome + dozens of accessory proteins)
Mirror tRNAs and all 20 aminoacyl-tRNA synthetases
Mirror membrane synthesis machinery
Integration into a viable synthetic cell chassis (which doesn’t exist even for normal chirality)
The CRS report notes this would require “Human Genome Project–scale effort and funding” (the HGP took ~13 years and nearly $3.8 billion).
Ting Zhu, the leading researcher in this field, acknowledges:
“Creating a mirror-image (or even natural-chirality) cell from scratch would require unprecedented technologies and vast resources that are currently unavailable.”
What people are actually working on right now
Today’s field is still overwhelmingly component-level mirror biology—not “building a mirror bacterium.” The active work tends to cluster around:
Making bigger mirror proteins reliably (long sequences, higher yields, fewer synthesis failures).
Mirror nucleic-acid toolchains (copying L-DNA, transcribing to L-RNA) at higher fidelity/lengths.
Translation-system parts (tRNAs, aaRS enzymes, ribosomal components) needed before anything could be self-replicating.
Cell-free systems and synthetic-cell “chassis” research (still hard even for normal chirality).
Safety/defense work (detection, immune interaction assays, and countermeasure plausibility).
This is why “moratorium” in practice is about not pushing the final integration step into a self-sustaining organism—not banning the whole domain.
CRS explicitly frames mirror life as not technically feasible now, “more than 10 years away” (per some scientists), and notes the December 2024 Science article + technical report calling for a moratorium.
The Opportunity Cost: What We’d Lose From a Moratorium
Before evaluating whether to halt this research, we need to honestly quantify what’s at stake. The potential benefits are substantial, likely understated (with unknown upside tail potential) and worth highlighting.
The Stability Problem: Why Mirror Matters
Market-size reports on “peptide therapeutics” vary widely, so rather than rely on opaque estimates, we can anchor the magnitude in audited revenue. In 2024, Novo Nordisk reported DKK 149,125 million in sales of GLP‑1-based products for type 2 diabetes (including Ozempic® at DKK 120,342 million), and DKK 65,146 million in obesity-care products (Wegovy®/Saxenda®). (NovoNordisk, 2024)
Against that backdrop, even modest reductions in the time/cost/complexity of making peptides long‑acting—or the ability to create stable peptide modalities that don’t require extensive chemical “workarounds”—can be economically and clinically meaningful.
The half-life catastrophe:
Native GLP-1 (the hormone behind Ozempic/Wegovy): half-life of ~1.5-5 minutes (PubMed)
After extensive chemical modification (lipidation, amino acid substitutions): Semaglutide achieves ~165 hours (7-day half-life) (FDA)
The modifications required to achieve this took decades of optimization and add manufacturing complexity
What mirror molecules offer:
D-peptides are intrinsically protease-resistant without complex modification
L-RNA aptamers (Spiegelmers) remain stable 60+ hours in human plasma at 37°C
In one study, an L-peptide lost ~90% activity after 8 hours in serum; the D-version was “mainly unaffected”
L-DNA stored information remained amplifiable and sequenceable after 1 year in pond water; natural D-DNA was undetectable after 1 day
The drug development implications:
~90% of drug candidates fail clinical trials (est. cost per failed drug: ~$800M-$1B+) (HHS)
A major failure mode is poor pharmacokinetics (ADME properties)
Mirror molecules could bypass years of stability optimization
Drug development attrition is extremely high, and pharmacokinetics / stability constraints are a common failure mode—especially for peptide modalities—driving years of iterative chemistry to extend half-life and reduce degradation.
Specific Benefits by Application
1. Cancer Therapy
NOX-A12 (olaptesed pegol), an L-RNA aptamer targeting CXCL12, showed remarkable results in Phase IIa trials for relapsed/refractory chronic lymphocytic leukemia:
96% overall response rate (combination with bendamustine/rituximab)
14% complete response rate
82% partial response rate
Reduction of lymphadenopathy ≥50% in 14/21 evaluable patients
Well-tolerated safety profile
For context: CLL remains incurable with conventional chemoimmunotherapy. Residual disease persists in bone marrow because cancer cells hide in protective stromal niches. NOX-A12 flushes them out by neutralizing CXCL12, the chemokine that attracts and shelters them.
2. Antibiotic Resistance
Mirror antimicrobial peptides (AMPs) could slow evolution of antibiotic resistance. Natural AMPs produced by hosts fight bacteria, but bacteria evolve resistance.
D-amino acid AMPs:
Retain antimicrobial activity
Resist degradation by bacterial proteases
May be harder for bacteria to evolve resistance against (would require evolving mirror proteases)
With antibiotic resistance killing 1.27 million people annually and projected to cause 10 million deaths/year by 2050, this isn’t theoretical.
3. Data Storage
DNA data storage density can exceed hard drives by orders of magnitude.
But natural DNA degrades. L-DNA demonstrated:
1 year stability in pond water (vs. <1 day for D-DNA)
Chiral steganography: hiding messages readable only with mirror polymerases
Potential for millennium-scale archival storage
IDC predicts the Global Datasphere grows from 45 ZB (2019) → 175 ZB (2025)
4. Industrial Enzymes
Mirror enzymes could:
Degrade plastics at scale. OECD estimates global plastics production reached ~460 Mt in 2019; only ~9% of plastic waste was recycled, while ~22% was mismanaged/uncontrolled or leaked into the environment.
Function in environments containing natural proteases
Process mirror substrates for pharmaceutical manufacturing
5. Basic Science
Understanding why life chose one chirality over its mirror could illuminate:
Origins of life
Possibility of alternative biochemistries
Fundamental constraints on living systems
6. Drug Discovery Platform (Mirror-Image Phage Display)
D-proteins aren’t just “products”—they enable a pipeline for discovering protease-resistant D-peptide drugs against normal (L) human targets.
Synthesize the mirror (D) version of a disease-relevant protein target
Run mirror-image selection / phage display against that D-target to find binders
Flip the winning binders into D-peptides → they bind the natural (L) target, but with built-in protease resistance
This matters because it turns “making D-proteins” into a reusable drug-discovery engine, especially for “hard targets” (protein–protein interactions) where small molecules struggle.
7. Biomanufacturing Platform (The Contamination / Phage Angle)
Mirror organisms would be a production chassis. If mirror microbes are largely invisible to normal phages/predators, you’re not just getting “mirror proteins”—you’re getting a potentially more robust fermentation platform where fewer things can crash the run.
That matters because a lot of real-world biomanufacturing pain is boring stuff like contamination and batch failures. A mirror chassis could mean:
Fewer phage wipeouts / fewer “the fermentor died” disasters (higher uptime)
Cheaper scale once you’re in the kg → ton regime
A new production paradigm for mirror enzymes/proteins that chemistry just can’t touch at commodity scale
Can We Get These Benefits Without the Risks?
I think so, and so do those pushing the mirror life moratorium.
The Safe Path: Chemical Synthesis of Mirror Components
Michael Kay (University of Utah) offers some insight:
“Mirror molecules that are created chemically cannot self-replicate, and therefore pose none of the risks of a mirror bacterium.”
Current capabilities:
Automated fast-flow peptide synthesis (AFPS) can produce proteins up to 164 amino acids in hours
12 D-proteins synthesized in single runs (nearly 1/3 of all D-proteins ever made)
Mirror Pfu polymerase (775 amino acids) was chemically synthesized via fragment assembly
L-RNA aptamers can be produced at pharmaceutical scale
What chemical synthesis can deliver:
D-peptide therapeutics (any length achievable through ligation)
L-RNA/L-DNA aptamers and storage molecules
Mirror enzymes for industrial applications
All the therapeutic Spiegelmers currently in trials
What chemical synthesis cannot efficiently deliver:
Massive quantities of complex mirror proteins at low cost
Potentially: mirror ribosomes (though not obviously needed for most applications)
Does AI Close the Gap?
A reasonable objection: if AI can accelerate conventional peptide optimization, does the “intrinsic stability” advantage of mirror molecules still matter?
What AI can now do:
AlphaFold3 predicts peptide-protein interactions with high accuracy
ML models predict peptide half-life from sequence (R² = 0.84-0.98 in recent work) (Briefings in Bioinformatics, 2024)
Generative AI designs novel peptides with desired properties
One 2025 study designed 10,000 de novo GLP-1 receptor agonists computationally, validated 60, and found two with half-lives ~3x longer than semaglutide in vivo
The honest assessment:
AI dramatically accelerates optimization of L-peptides—but doesn’t eliminate the underlying problem.
Here’s the key distinction:
Approaches:
Mirror peptides: Get intrinsic protease resistance from D-amino acid backbone—no modifications needed
AI-optimized L-peptides: Get predicted modifications that improve stability—but still need to add lipidation, PEGylation, or other modifications that increase manufacturing complexity
Even the best AI-designed L-peptide requires additional modifications (lipidation, PEGylation, non-natural amino acids) to achieve extended half-life.
AI accelerates finding which modifications work—but you’re still adding manufacturing complexity that D-peptides avoid entirely.
The narrowing gap:
For many therapeutic applications, AI-optimized L-peptides may be “good enough” in practice
Example: An AI-designed GLP-1RA reported ~3x longer half-life than semaglutide with comparable weight loss in an obesity mouse model (Wei et al., 2025).
Manufacturing costs for modifications are falling
Where mirror molecules may still win:
Applications requiring extreme stability (e.g. data storage for 1 year vs. 1 day)
Cost-sensitive industrial applications (simpler = cheaper at scale)
Novel targets where extensive L-peptide optimization isn’t already done
Situations where added modifications cause problems (immunogenicity, aggregation)
Bottom line: AI narrows the gap but doesn’t close it. Mirror molecules offer intrinsic stability; AI helps you engineer stability into L-peptides. For most therapeutic applications, AI-assisted L-peptide optimization may be sufficient. For applications requiring extreme stability or cost-sensitive scale, the mirror advantage persists.
The Cost of the Safe Path
Chemical synthesis of mirror feedstocks (D-amino acids, L-nucleotides, L-sugars) is expensive. For a simple anchor: catalog pricing puts D-(+)-glucose at tens of dollars per kilogram, while L-(–)-glucose is hundreds of dollars for a few grams—i.e., ~10³×+ price differentials between enantiomers are normal.
A 775-amino-acid protein requires laborious multi-fragment assembly.
The manufacturing argument for mirror organisms: biological production could achieve economies of scale that chemistry cannot. A mirror bacterium could, in theory, churn out mirror proteins the way E. coli produces insulin today.
But here’s the critical question the moratorium advocates dodge: How much manufacturing efficiency are we sacrificing, and does it actually matter?
Quantifying the slowdown: AI is downstream acceleration. Mirror organisms are platform acceleration. Regulation is the handbrake on both. The cost of a moratorium isn’t “mirror science gets 3× slower.”
For most therapeutic-scale mirror molecules, chemistry/cell‑free already works.
The real cost is that you take a bunch of potential industrial mirror-biology applications and push them over a feasibility cliff.
The unit isn’t “×”; it’s “does this category happen at all, and how many years does it get delayed?”
A practical way to think about it:
Drug scale (mg → g): Mostly not blocked by a moratorium on mirror organisms. Slower iteration + higher cost, but doable.
Industrial scale (kg → tons): This is where organisms matter. Without a living chassis, a lot of applications are effectively “no” (or “not for a long time”) because you can’t brute-force ton-scale production with boutique chemistry forever.
Iteration-speed cost (the search bottleneck): A living chassis doesn’t just make production cheaper, it can make exploration faster. Replicating systems enable biology-style selection and evolution loops; purely chemical/cell-free workflows usually can’t match that throughput once sequences get long. So a moratorium’s cost isn’t only “tons are off the table,” it can also be “some discovery loops become impractical.”
If you want a back-of-envelope way to quantify the opportunity cost, use:
Opportunity cost ≈ (annual value if it works) × (years delayed) × (probability it would have worked).
Example: If you think there’s a 20% chance a $10B/year platform exists, delaying it 15 years is $30B expected value foregone (0.2 × 10 × 15).
The honest answer: Chemical synthesis is improving rapidly. Automated flow systems are driving costs down. For therapeutic applications, the quantities needed are small (milligrams to grams, not tons). The manufacturing argument was stronger 20 years ago than today.
Where it might still matter:
Industrial-scale mirror enzymes (e.g., for plastic degradation)
Any application requiring metric tons of mirror protein
Applications we haven’t thought of yet
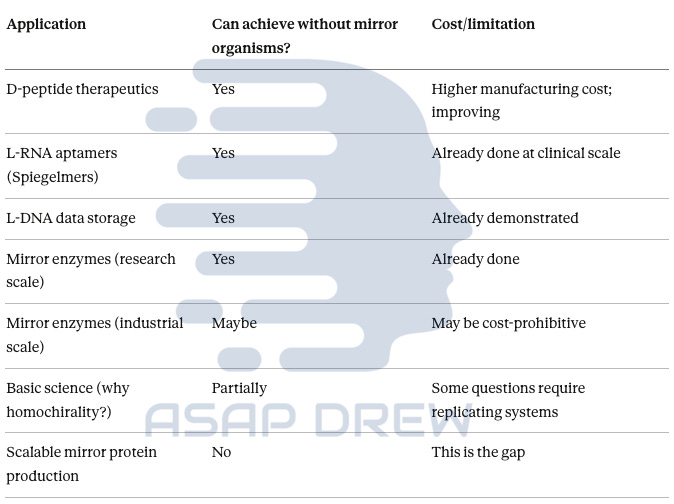
The Workaround Summary
Workaround? (Yes, No, Maybe, Partial)
D-peptide therapeutics: YES—chemical synthesis delivers clinical-grade material; costs improving with automation
L-RNA aptamers (Spiegelmers): YES—already in Phase II trials; chemical production works
L-DNA data storage: YES—demonstrated with 1-year stability; no organisms needed
Mirror enzymes (research): YES—775-amino-acid polymerase already synthesized chemically
Mirror enzymes (industrial): MAYBE—depends on scale required; may be cost-prohibitive without biological production
Basic science questions: PARTIALLY—some origin-of-life questions may require replicating systems
Scalable mirror protein manufacturing: NO—this is the genuine gap that mirror organisms would fill—and it’s not a “3x” issue (ton-scale applications off the table)
Directed evolution / selection in a fully mirror system: NO — without a replicating mirror chassis, you can’t do end-to-end “biology-speed” evolution in the mirror domain.
Mirror-image ligand discovery (D-protein targets): YES—doesn’t require mirror organisms; it mainly requires the ability to synthesize D-protein targets (which is improving fast).
The December 2024 Moratorium Call: What They Actually Argued
On December 12, 2024, 38 prominent scientists published in Science calling for a moratorium. Authors included two Nobel laureates (Greg Winter and Jack Szostak), George Church, J. Craig Venter, and leading experts across immunology, ecology, evolutionary biology, and biosecurity.
The accompanying 299-page technical report represents the most comprehensive risk assessment to date.
Their Core Claims
Mirror bacteria could:
Evade many immune defenses across humans, animals, and plants due to chirality-dependent recognition mechanisms
Escape natural predation from bacteriophages and other organisms
Persist environmentally as an invasive species with few natural checks
Resist countermeasures (and even if some antibiotics work against some mirror targets, ecosystem-scale deployment is the real impossibility)
What They Called For
A halt on research aimed at creating mirror bacteria
Funders to refuse support for such work
Global dialogue on governance frameworks
Translation: This isn’t “ban mirror molecules.” It’s “don’t push on the one threshold that changes everything”: a self‑replicating mirror microbe. Under that framing, mirror-bio work doesn’t automatically shut down—it re-routes into safer lanes.
In-scope to pause: projects whose goal is a self‑replicating mirror bacterium / mirror cell / mirror chassis
Still on the table: chemically synthesized mirror peptides/proteins, Spiegelmers/aptamers, L‑DNA data storage, mirror enzymes as tools, cell‑free systems, and “risk‑tightening” experiments/models
Kate Adamala, a synthetic biologist at the University of Minnesota and report co-author, switched from working on mirror cells to opposing their creation:
“We should not be making mirror life. We have time for the conversation.”
The “Immune Evasion” Logic: Mechanistic Analysis
Why the Concern Is Mechanistically Plausible
Many immune defenses rely on chiral molecular recognition:
Antigen presentation: MHC molecules process protein antigens through proteolytic cleavage before presenting peptide fragments to T cells. Polypeptides built exclusively of D-amino acids resist this processing because peptidases are stereospecific.
Antibody responses: Studies found D-enantiomer peptides produce unusual IgG3-dominated responses and limited cross-reaction with L-peptides “probably due to different sterical conformations of the MHC-antigen-T cell receptor complexes.”
T cell recognition: D-peptides have two mechanisms for “immunosilencing”: protease resistance that hinders MHC presentation, and altered backbone conformations affecting recognition.
This is the same logic that makes mirror molecules useful as therapeutics—they persist precisely because the body can’t degrade them. The sword cuts both ways.
Why the Concern May Be Overstated
A substantive counterpoint in Science eLetters discussion of “Confronting the Risks of Mirror Life” argues the technical report left critical issues undiscussed.
The Glycan Blind Spot
In plain English: Even if “mirror proteins” evade lots of immune detection, the immune system also recognizes sugary coatings on microbes, and those coatings are already extremely diverse—so “totally invisible” is likely an overstatement.
A 2025 Science eLetter “Remember the Glycans: Consideration of Glycans in Evaluating the Threat of Mirror-Image Life Forms” response signed by 30+ glycoscientists (including Nobel laureate Carolyn Bertozzi) argues the original mirror-life risk framing underweighted the “third pillar” of biomolecules: carbohydrates.
Core point: Our immune systems already deal with “weird” microbial glycans (including L-sugars and unusual stereochemistry) because bacterial cell surfaces are glycan-heavy and wildly diverse.
So “mirror bacteria = completely novel surfaces = totally invisible” is not obviously the default.
Concrete anchors:
Anti-L-rhamnose antibodies show up broadly in human serum because L-rhamnose is a common microbial sugar/epitope humans encounter. (R)
Human intelectin-1 is an example of innate recognition that keys off structural motifs (e.g., terminal exocyclic 1,2-diols) that are microbial and not just “chirality-dependent lock-and-key.” (R)
Implication: Mirror bacteria might still trip meaningful immune recognition through glycan biology, even if D-proteins and mirror peptidoglycan create serious blind spots elsewhere. In other words: this doesn’t kill the immune-evasion argument — it mainly kills the “totally invisible” strongest-form version.
This is a mitigation factor for immune evasion, not a refutation of the broader risk case — predator evasion + invasive-species dynamics could still dominate even if glycan recognition partially works.
Not all immunity is chirality-dependent:
Complement system activation (some pathways)
Physical barriers (skin, mucus)
Some antimicrobial peptides that act via membrane disruption rather than specific binding
Phagocytosis (physical engulfment)
Pathogenicity requires more than immune evasion:
Efficient growth at body temperature
Nutrient acquisition (complex in a mirror-normal world)
Functional virulence factors (toxins, adhesins)
Intracellular invasion mechanisms may fail with chirality mismatch
Natural precedents for chirality diversity:
Bacteria already produce D-amino acids in their cell walls
Some lipopolysaccharides contain both D- and L-rhamnose
Natural immune systems cope with significant chiral diversity
The UK Government Office for Science (2025) notes chirality mismatch:
“Might also prevent some pathogenic mechanisms (e.g., intracellular invasion of host cells) from occurring.”
The Honest Assessment
The immune evasion concern is plausible and mechanistically grounded, but the degree of evasion is legitimately uncertain.
We cannot know whether mirror bacteria would be:
Completely invisible to immunity (worst case)
Partially evading but still somewhat controlled
Able to grow but unable to cause disease due to incompatible virulence mechanisms
This uncertainty is itself an argument for caution—but it’s not the same as knowing the threat is severe.
Could Mirror Bacteria Actually Compete in Our World?
Arguments That Mirror Bacteria Could Survive and Spread
Achiral nutrition exists:
Many bacteria can grow on mineral nutrients and CO₂
Autotrophs could potentially establish themselves
Some organic nutrients are achiral or racemically available
Predator evasion:
Bacteriophages rely on chiral surface receptors—mirror bacteria would be invisible to all natural phages
This is “absolutely 100 percent sure” according to Szostak (Harvard Mag)
Phages are a major cause of bacterial cell death in nature
Invasive species dynamics:
Species spread by being unchecked, not by being intrinsically fitter
Kudzu and zebra mussels aren’t “better”—they’re just uncontrolled in new environments
A mirror bacterium wouldn’t need to outcompete—just survive without predation
Arguments That Mirror Bacteria Might Struggle
Nutritional constraints:
Most natural environments have chiral organic nutrients
Converting D-sugars to L-sugars requires additional metabolic machinery
Competition for achiral nutrients would be against optimized natural organisms
Growth limitations:
Mirror bacteria might grow slowly due to nutrient limitations
Slow growth reduces both spread and pathogenicity
Unknown incompatibilities:
Some ecological control mechanisms might work regardless of chirality
We simply don’t know what we don’t know
The Invasive Species Framing
The technical report’s most compelling argument: mirror bacteria could behave like invasive species.
Key insight: Invasive species don’t need to be “better”—they just need to be unchecked. Zebra mussels have devastated Great Lakes ecosystems not because they outcompete native species in a fair fight, but because their natural predators didn’t make the trip from the Black Sea.
Mirror bacteria would have no natural predators, period. No phages. No nematodes evolved to eat them. No protists that recognize their surfaces.
This framing shifts the question from “would mirror bacteria be super-organisms?” to “would removing all predation pressure allow them to proliferate?” The latter is much more plausible.
Quantifying Risk of Mirror Life / Organisms
Near-Term Risk Assessment (Next 10 Years)
10Y Risk Assessment:
Mirror bacteria created (10yr): <5% probability, high confidence—CRS says “more than 10 years away” with HGP-scale resources required
Deliberate release: <1%, high confidence—no organism exists to release
Mass-casualty pandemic: ~0%, very high confidence—can’t have outbreak from nonexistent pathogen
Human extinction: ~0%, very high confidence—prerequisite scenarios haven’t occurred
Conditional Risk Assessment (If Mirror Bacteria Were Created and Released)
These estimates assume a generalist, environmentally capable mirror bacterium enters the wild:
What happens if created and released?
Dies out: 30-60%, medium confidence—nutrient constraints and competitive disadvantage are plausible
Localized persistence: 20-40%, medium confidence—could survive in niches via predator evasion
Severe ecological disruption: 10-30%, low-medium confidence—invasive species dynamics are real but degree uncertain
Mass-casualty pandemic: 1-15%, low confidence—requires efficient human growth AND transmission AND pathogenicity
Human extinction: <0.5%, low confidence—requires compounding worst-cases; no historical precedent for single-pathogen extinction
The Meta-Point
The probability distribution is dominated by whether mirror organisms are built and released (a governance decision), not the downstream biology (which cannot be empirically determined without creating the risk).
The conditional probabilities are largely unfalsifiable without running the experiment—which is precisely what makes this a precautionary situation.
Can We Test Risks Without Creating Mirror Organisms?
What We Can Test
The UK roundtable acknowledged: “we won’t understand many risks… unless it comes into being.” But some component-level testing is feasible:
How can we get more risk information via safe experimentation?
Innate immunity: In vitro assays with D-peptide particles on immune cells—would show if pattern recognition receptors detect mirror molecules
Complement system: Biophysical assays on mirror lipid vesicles—tests if complement pathway activates on mirror surfaces
Antimicrobial peptides: Membrane disruption assays on mirror membranes—reveals if AMPs work through chirality-independent physical mechanisms
Antibiotics: Binding assays with mirror enzyme targets—already being done computationally for drugs like amoxicillin
Metabolism: Computational modeling of mirror metabolic networks—predicts growth constraints without organisms
What Testing Cannot Resolve
Emergent properties require complete organisms:
Competitive fitness in real ecosystems
Evolutionary adaptation potential
Actual transmission dynamics
Long-term persistence and spread
This irreducible uncertainty is central to the precautionary argument. We can do component testing, but we cannot fully characterize organism-level risks without creating the organism.
Historical Precedents: How Have Similar Situations Played Out?
Recombinant DNA (1975 Asilomar)
Scientists voluntarily paused recombinant DNA research pending safety assessment. The pause lasted ~2 years. Result: safety protocols were developed; research resumed; biotechnology industry flourished.
Lesson: Voluntary moratoriums can work when the scientific community is aligned and the technology requires institutional resources.
Gain-of-Function Research (2012-2017)
H5N1 experiments that could enhance transmissibility sparked debate. NIH imposed funding pause in 2014. Research resumed in 2017 with enhanced oversight.
Lesson: Government funding restrictions work for funded research; they don’t prevent well-resourced actors operating outside oversight.
Human Cloning
Near-universal ban achieved through combination of professional ethics, funding restrictions, and some national laws. No successful human reproductive cloning has occurred.
Lesson: Strong social consensus against crossing certain lines can be effective—but requires genuine consensus.
George Church’s Skepticism
Church, a moratorium co-author, notes historical attempts at prohibition have sometimes backfired:
“A moratorium is a semi-voluntary thing, but it’s something that only the good guys are going to sign up for.”
Federal funding bans on stem cell research and recombinant DNA sparked private investment that continued the work outside oversight. The question isn’t whether good actors will comply—it’s whether compliance matters if bad actors don’t.
Ting Zhu’s Counterargument: The Case Against Premature Bans
The leading researcher in mirror molecular biology published his perspective in Nature (commentary), arguing for dialogue over prohibition:
“Amid the race to take action, it is important not to let concerns and anxieties obscure our judgement of the underlying unknowns.”
His Key Points
The distinction matters: Existing mirror component research is valuable and safe. Only self-replicating mirror organisms pose the hypothesized risks.
Historical caution: Banning technologies before understanding them has prevented major benefits:
Alternating current was initially banned in some jurisdictions
Recombinant DNA fears were ultimately manageable
We don’t know what benefits we’d forgo
Benefits at stake:
More effective drugs with intrinsic stability
Enzymes that degrade environmental pollutants
Robust DNA data storage resistant to biodegradation
His position: Support “establishing ethical boundaries” through “comprehensive assessment of near-term challenges and long-term risks across multiple disciplines”—but oppose premature bans on basic research.
The Response
Adamala responds:
“He’s said he’s not going to build a living mirror cell, and that’s good enough for me.” (This appears to be based on private communication; I have not seen a public statement from Zhu committing to this.)
This highlights the current equilibrium: leading researchers seem willing to stop short of creating self-replicating organisms, even without formal prohibition.
The question is whether this informal consensus is durable.
How Would a Moratorium Be Enforced?
There is no binding global moratorium today.
What exists is a scientific call for one, plus ongoing governance efforts.
Current and Proposed Mechanisms
Moratorium mechanisms:
Funding restrictions: Being urged—NSF previously funded $4M for mirror cell research; this may change
Research norms: Scientific community appears aligned against creating mirror organisms
Publication controls: Could restrict knowledge spread; controversial and potentially ineffective
International coordination: Mirror Biology Dialogues Fund organizing conferences (Paris, Manchester, Singapore, etc. — more planned)
DNA synthesis screening: Could flag orders for mirror components; not currently implemented
The Enforcement Problem
The CRS acknowledges:
“If not implemented equally across the globe, a moratorium in the United States could encourage scientists to move to other countries.”
Additional challenges:
No clear regulatory authority over technology that doesn’t exist yet
Dual-use overlap with legitimate therapeutics research
State-level actors may not be deterrable
Technology will eventually mature regardless
The AI Wild Card: Accelerated Design and Evasion of Safeguards
One risk the moratorium advocates haven’t fully addressed: AI is rapidly changing the landscape for both legitimate research and potential misuse.
AI Could Accelerate Mirror Organism Development
The same AI tools transforming drug discovery could accelerate mirror biology:
Protein design at scale:
AlphaFold3 and RoseTTAFold predict 3D structures from sequences
Generative AI can design novel proteins with specified functions
Automated flow synthesis can rapidly produce designed proteins
The timeline compression problem:
Tasks that once required years of graduate student labor can now be done in days
The CRS estimate of “10+ years” to mirror bacteria assumes current development pace
AI could compress this dramatically
AI Could Help Circumvent Safeguards
More concerning: AI tools may help bad actors evade biosecurity screening.
The “paraphrase” problem (October 2025): A Microsoft Research study found AI protein design tools can “paraphrase” toxins—generating sequences that preserve function while evading screening:
Researchers used EvoDiff to generate thousands of synthetic ricin variants
These variants preserved active sites and structure but had novel sequences
Many passed through standard DNA synthesis screening software
DNA providers Twist Bioscience and IDT have since updated their screening algorithms
The core vulnerability: Current biosecurity screening relies on sequence homology to known threats. AI-designed proteins can achieve the same function with unrecognizable sequences. As one expert noted: “This screening relies on sequence similarity to known hazards and could be undermined by the ability to create novel sequences with similar functions but undetectable sequence homology.”
Implications for mirror biology:
AI could design optimized mirror proteins without years of trial-and-error
Novel mirror sequences wouldn’t match any database of “sequences of concern”
Screening would need to shift from sequence-matching to function-prediction
The Rogue Actor Scenario
George Church’s concern—”only the good guys sign up for” moratoriums—becomes more acute with AI:
Lowered barriers:
AI reduces the expertise required for sophisticated biological design
Automated labs reduce the hands-on skill required
The combination could enable smaller, less accountable actors
Governance lag:
AI capabilities advance faster than policy
The 2024 Framework for Nucleic Acid Synthesis Screening doesn’t address AI-designed sequences
No international coordination on AI-biology intersection
The counterargument: Creating a functional mirror organism still requires massive wet-lab infrastructure, not just in silico design. AI can’t yet design fully replicating systems from scratch. The “Paraphrase Project” showed evasion of screening, but also prompted rapid patches to screening software.
The Honest Assessment
AI both increases the urgency for governance and makes governance harder:
Faster capability arrival: What’s “10+ years away” could become 5 years
Harder to screen: Novel sequences evade homology-based detection
Harder to enforce: Smaller actors, distributed work, less institutional oversight
But also better defense: AI can improve screening, detection, and countermeasure design
This is a reason to accelerate governance discussions, not abandon them. But it also suggests that moratoriums premised on “we have time” may be more fragile than advocates assume.
Could Mirror Life Already Exist?
Why It Almost Certainly Doesn’t
Evolutionary impossibility: The Science paper states mirror life “cannot arise from existing life because evolution proceeds incrementally.” Flipping chirality requires simultaneously inverting an entire molecular system—there’s no viable intermediate.
No detected anomalies: Environmental chemical analyses show strong homochiral dominance. If mirror life were widespread, we’d see anomalous L-sugars and D-amino acids in environmental samples.
Detection gap: Standard genomics methods (PCR, sequencing) use natural-chirality probes that wouldn’t bind mirror templates. The UK recommends developing chirality-targeted biosurveillance—implying current methods would miss mirror life if it existed.
The Honest Position
“Mirror bacteria are widespread on Earth” = extremely low probability (high confidence)
“A tiny isolated pocket exists undetected” = not strictly impossible, but very unlikely given origin constraints and lack of anomalies
We can be quite confident mirror life doesn’t exist naturally, while acknowledging we haven’t systematically looked.
The Critical Question: Is the Moratorium Actually the Right Call?
Having laid out the evidence, let me offer a more critical assessment than the consensus position.
Arguments the Moratorium Is Correct
Irreversibility: If mirror bacteria spread, there’s no recall. Unlike most technological risks, the worst case cannot be undone.
Uncertainty favors caution: We cannot characterize organism-level risks without creating the risk. The precautionary principle applies.
Limited downside: Most benefits of mirror biology can be achieved through chemical synthesis. The gap (scalable biological production) isn’t obviously essential.
Time to prepare: A moratorium buys time to develop detection, countermeasures, and governance frameworks before capability arrives.
Scientific consensus: 38 experts including Nobel laureates, plus leading institutions (J. Craig Venter Institute, etc.), have endorsed this position.
Arguments the Moratorium Is Wrong or Premature
Opportunity cost underestimated: $100+ billion market; drugs that could save millions of lives; solutions to antibiotic resistance. The moratorium advocates handwave this as “achievable through chemical synthesis” without quantifying the efficiency loss.
Manufacturing matters: For industrial-scale applications (plastic degradation, enzyme production), chemical synthesis may never be economically viable. We’re potentially foreclosing entire application categories.
Only good guys comply: If the technology becomes feasible, state-level bad actors won’t be deterred by scientific consensus. A moratorium may just ensure Western researchers aren’t positioned to respond.
Risks may be overstated: The immune evasion argument is plausible but unproven. Natural chirality diversity, nutritional constraints, and incompatible virulence mechanisms may limit actual pathogenicity. We’re making policy based on theoretical worst-cases.
Historical track record: Voluntary moratoriums on recombinant DNA didn’t prevent the biotechnology revolution—they just delayed and improved it. Why expect worse from mirror biology?
Basic science value: Some fundamental questions about life’s origins and alternative biochemistries may require studying replicating systems. Are we sure we want to foreclose this?
My Assessment
I’ve done a lot of thinking about this… and this is where I landed.
I currently lean toward a targeted, time-limited moratorium (reviewed annually) on attempts to create self-replicating mirror organisms (not a ban on mirror molecules, cell-free systems, or defensive research).
The decision is asymmetric:
The downside includes a low-probability but potentially irreversible fat-tail catastrophic failure mode (accident, misuse, or ecological persistence)
Much of the upside attributed to “mirror life” appears substitutable via: (1) mirror components made without self-replication, or (2) entirely distinct competing technologies.
Even if some substitutes are less efficient today, automation and AI are likely to make many workarounds more practical over time.
A pause mainly buys time to: (1) clarify which benefits, if any, truly require living self-replication, (2) mechanistically map plausible failure modes, and (3) build detection, biosurveillance, containment standards, and countermeasures—capabilities that may improve substantially as AI and tools advance.
The strongest objection is “kicking the can”: if the capability is likely to emerge eventually, delaying responsible actors could increase the chance that a less responsible defector gets there first.
That’s why the goal isn’t indefinite prohibition; it’s stage-gating—pause broad, unconstrained attempts at self-replication while accelerating safer paths, defenses, and governance, and only revisiting the threshold step if a large, non-substitutable benefit and a robust containment story become clear.
What Should We Believe?
High Confidence
Mirror bacteria do not currently exist and are technically infeasible for at least the next decade
Mirror components have substantial value in therapeutics, with clinical evidence of safety and efficacy
The immune evasion mechanism is plausible based on well-established chirality-dependent biology
Most therapeutic benefits can be achieved through chemical synthesis of mirror components
Near-term extinction risk from mirror life is negligible because the prerequisite organisms don’t exist
Medium Confidence
Creating mirror bacteria will eventually become feasible as enabling technologies advance (10-30 year timeframe)
Some immune defenses would fail against mirror organisms (those dependent on chiral recognition)
Some defenses might still work (physical/mechanical mechanisms less dependent on chirality)
Nutrient constraints could limit mirror organism viability in complex environments
A moratorium would slow but not prevent eventual capability emergence
Low Confidence / Genuine Uncertainty
Whether mirror bacteria would thrive or die in real ecosystems
Exact pathogenicity and transmission potential in humans/animals
Effectiveness of potential countermeasures at scale
Conditional extinction probability given creation and release
Whether foreclosing biological production forecloses essential applications
Whether good-actor moratoriums prevent bad-actor development
The Policy Implication
The strongest case for precaution is not certainty of catastrophe, but irreducibility of uncertainty combined with potential irreversibility.
We cannot run the experiment to find out without potentially triggering the outcome we’re trying to avoid.
But the case isn’t as clear-cut as advocates present it. We are trading concrete benefits (better drugs, industrial applications, basic science) for reduced probability of a theoretical worst-case whose likelihood we cannot estimate.
Dilemma? Yes. Paralysis? No. The sensible move is to pause the self-replication threshold, build surveillance and countermeasures, and demand a clear non-substitutable upside before reopening the door.
Here is a preliminary biodefense framework I created: Mirror Life Biosecurity Framework: Defense Protocol for Mirror Bacteria.